5 Ways Gene Therapy Companies are Transforming Rare Disease Cures

Contributing Author: Gina Hagler
Historically, developing rare disease cures and treatments has been challenging. The small, globally dispersed patient population made it difficult and expensive to bring together enough individuals for a clinical-stage trial. The small patient population of fewer than 1% of the population also made it impossible to recoup the investment in research and development costs required for a cure utilizing the chemical-based approach of traditional pharmaceuticals. Additionally, the long research and testing phases left little time to recoup the money invested before the patent ran out.
Today, advances in remote trial technology and the financial benefits that come with an Orphan Drug Designation are helping to overcome these obstacles. This has led to the development of cutting edge new therapies, yet, there’s no escaping the fact that after decades of research, testing, and development, only 5% of the 7,000 known rare diseases have a cure. This abysmal success rate is due not only to the factors cited but also because 80% of rare diseases are genetic disorders. Traditional pharmaceuticals are not the best fit for developing new treatments for genetic errors. It is only by turning to biopharmaceutical companies that we are seeing significant progress in therapies for genetic disorders such as cystic fibrosis and sickle cell disease. Gene therapy companies are transforming rare genetic disease cures by using patients’ own genes. These pioneering treatments hold great promise for improving the lives of patients by significantly slowing the progression of a disease and sometimes providing a durable - lasting - cure.
1. Genetic testing opens new possibilities
Researchers have long suspected that some diseases had a hereditary basis while others were due to mutations in an individual’s DNA. For hereditary diseases like cystic fibrosis and sickle cell disease, the faulty gene or sequence is in the germline. As a result, the potential for developing the disease is passed to each new generation. Today, we are not quite ready to perform gene editing that will affect future generations, but we can screen to determine if a couple carries the genes for known hereditary diseases and counsel them about the statistical outcome, as is the case with the BRCA gene and breast cancer. We can also test patients without a known hereditary component to determine if they have one of the 7,000 other known rare diseases, including those that are neurodegenerative. Since 80% of rare diseases are monogenic - due to a genetic error in a single gene - it is often possible to identify the faulty gene through sequencing. Unfortunately, until very recently in pharma, identifying and curing have been two different things.
In a 2015 paper, Evolution of Genetic Techniques: Past, Present, and Beyond, Asude Alpman Durmaz et al. wrote, “Due to rapid advances in genomic technologies, genetics analyses have become essential in clinical practice and research. During the past decade, a great stride has been made to unravel underlying mechanisms of genetic-related disorders.” With the increasing access and sophistication of genetic testing and diagnosis has come increased interest in and availability of tools to explore possible “fixes” for these genetic conditions through the use of gene therapy. (A timeline of significant events is included as a timeline in Fig. 1)
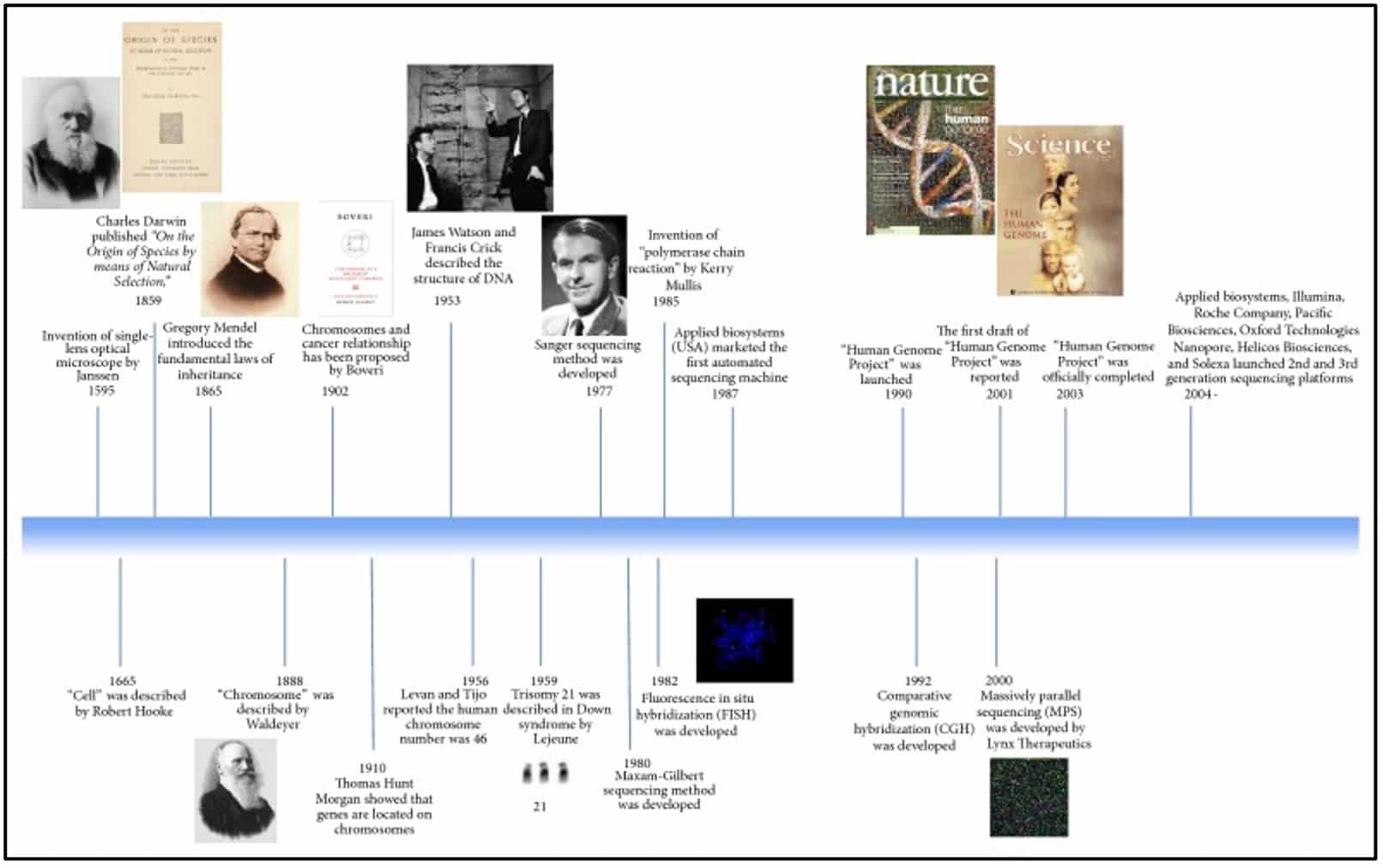
Fig. 1 Landmarks in genetic history Copyright © 2015 Asude Alpman Durmaz et al.
2. Promise of delay in disease progression, if not a durable cure, where there was once no hope
Gene therapy treatments are in their infancy. Early research has shown that it is now possible to introduce genes to take over the function of or “turn off” the faulty gene. “The ideal target for gene therapy [has been] Mendelian diseases caused by defined single-gene mutations, such as hemophilia or Duchenne muscular dystrophy. That has been its initial focus, bolstered by approvals of drugs such as Luxturna (for a type of Leber’s congenital amaurosis, an inherited blindness disorder) and Zolgensma (for spinal muscular atrophy). Now, gene therapy is taking on more complex, multigene diseases such as central nervous system diseases, neuropathic pain, sleep apnea, and cancer. Beyond correcting genes, gene therapy is revolutionizing cell-specific delivery of therapeutic proteins and synthetic drugs, as well as inspiring basic research into unanswered questions on immunogenicity.” [Sarkar, 2020]
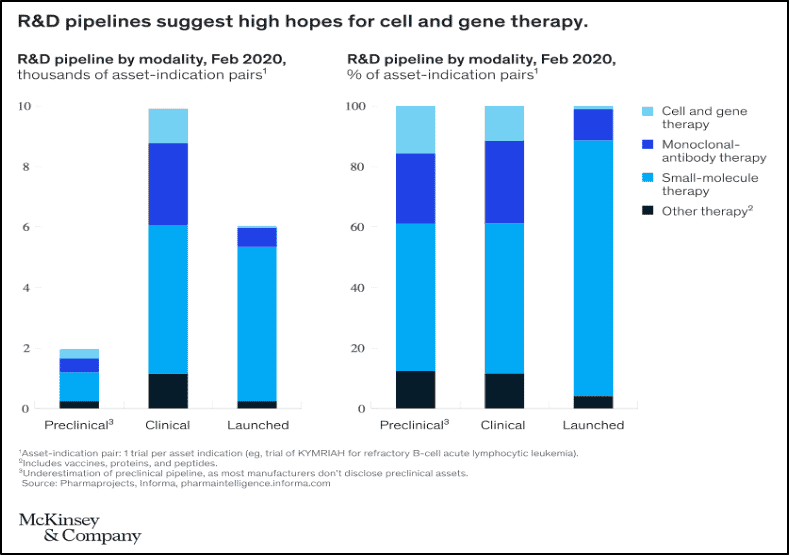
Fig. 2 [McKinsey & Company, 2020]
The progression of the diseases treated with Luxturna and Zolgensma have so far been arrested. Whether this will ultimately result in a “durable” cure that goes the distance won’t be known until more time has passed. Despite the fact that gene therapy is neither a sure thing nor completely safe, as seen in the death of two young pediatric patients last summer in an experimental trial for an, otherwise deadly, rare muscular disorder [Sarkar, 2020], hopes for gene therapy remain an important part of the future for biopharma. (Fig. 2)
3. Shorter research and development phases reduce the cost of development
The research and development phases of traditional pharmaceutical treatments take years and costs billions of dollars. By the time the drugs reach the market, there is not enough time to recover those costs while the drug is under patent and free from competitors. One of the benefits of gene therapy is a shorter development phase resulting from the nature of the research. With biopharma, researchers begin with a specific delivery mechanism, meaning there is no need for a drug discovery phase. With a delivery mechanism in hand, testing for efficacy can be done before formal trials are begun.
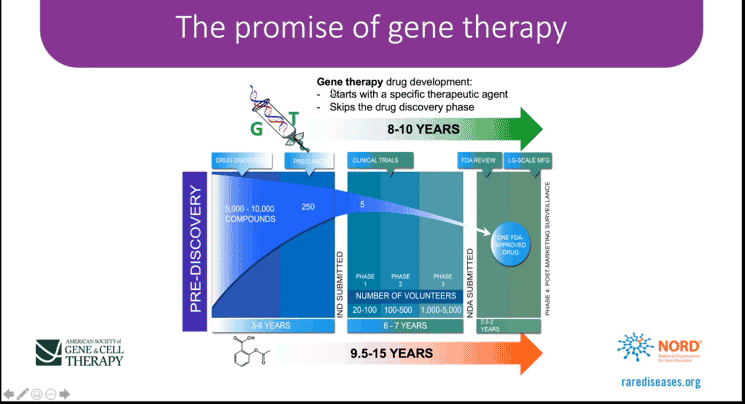
Fig. 3 [ASGCT/NORD, 2019]
According to a 2019 webinar on the promise of gene therapy presented by the American Society of Gene + Cell Therapy (ASGCT) and the National Organization for Rare Disorders (NORD), starting with a specific therapeutic agent and skipping the drug discovery phase reduces the time for development from 9.5 to 15 years to 8 to 10 years. (Fig. 3) This quicker route benefits both the pharmaceutical company and the patient. (Read more in 9 Reasons Treatment of Rare Disease Will be Big in 2025.)
4. The Orphan Drug Act provides benefits for drugs with Orphan Drug Status
Benefits that include tax credits, market exclusivity, and waiver of significant fees (Fig.4) are definitely an incentive.

However, the rising cost of R&D is one looming problem for traditional pharmaceuticals that will not be remedied by the Orphan Drug Act. A recent report by BioPharmaTrend on The Evolution of Pharmaceutical R&D Model [Buvailo, 2020] stated, “There is a plethora of analytics reports, including ones by Deloitte, DKV Global, and Ernst and Young, all pointing to the declining business performance of the pharmaceutical industry. They all convey a similar bottom-line message: The decline is not due to a lack of innovation (the innovations are growing). And not because sales are falling or markets are shrinking (revenues are growing in general, and the markets are expanding with the expanding and aging population). The key reason for the declining financial performance is the fact that research and development (R&D) costs are growing substantially faster over an average investment period than the actual revenues over the same period. This kills operational profits, leading to a decline in the overall business gain.” These increases in R&D costs will likely not impact gene therapy companies as dramatically due to their shorter R&D window.
5. Greater interest and FDA Guidances and approvals for rare diseases
With 80% of the 7,000 known rare diseases caused by a single gene, growth in the number of rare disease trials and FDA approvals are another indicator of the growing interest in gene therapy treatments for rare disorders.
In January 2020, FDA released six final guidance documents on gene therapy manufacturing and clinical development of products. It also released draft guidance, Interpreting Sameness of Gene Therapy Products Under the Orphan Drug Regulations. These guidances were released as a signal of strong support for the development of gene therapy products for the unmet medical needs of patients. The guidances provide answers to the questions asked by those developing gene therapy products and the orphan-drug designation. [FDA]
Chemical & Engineering News reported in 2019, the US Food and Drug Administration says it is preparing for a coming wave of experimental cell and gene therapies. By 2020, the agency expects it will see more than 200 applications a year requesting permission to begin cell and gene therapy trials. The agency already has more than 800 such applications on file and plans to hire some 50 clinical reviewers to handle the surge. [C&EN]
As of October 2020, FDA had approved five gene therapy products for six diseases. (Fig. 5) Earlier this year, the FDA announced that it “anticipates many more approvals in the coming years, as there are more than 900 investigational new drug applications for ongoing clinical studies in this area. [Sakar]
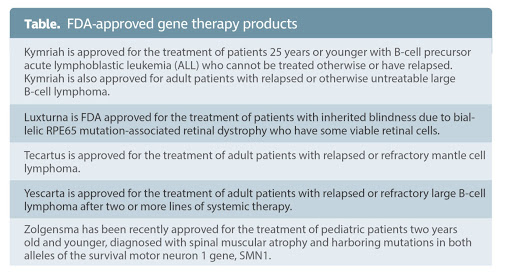
Fig. 6 [Sarkar, 2020]
Furthermore, FDA announced in May 2020, that it is establishing a rare disease clinical trials network to provide a more cooperative approach in supporting the drug development pipeline for rare diseases. [RF]
Conclusion
The ability to test the human genome and pinpoint genetic errors brought with it a desire to “fix” those errors. That desire went hand-in-hand with the drive to develop tools and methodologies that would make this possible through gene therapy. As gene therapy treatments came to be viewed as ideal for monogenic disorders, the push for human trials increased. The outcomes of these trials have largely shown that the progression of the diseases can be favorably impacted. With a shorter research and development time than that for traditional therapies, gene therapy has the advantage of a lower cost to market than traditional therapies. This is especially important because the patient population for these drugs is definitely smaller than that for the average disease. As another factor in reducing the net cost of bringing a gene therapy to market, the Orphan Drug Designation confers a number of financial benefits. The increased number of applications and trials is further evidence of a new approach to rare disease cures. The possibility of a cure for diseases for patients who were previously without hope, combined with reduced costs and greater FDA support, is bringing energy and momentum to gene therapy as a way to halt if not cure rare diseases thought to have a genetic basis. It is at the nexus of these factors that gene therapy companies are transforming rare disease cures.
Take Away
American Gene Technologies is dedicated to the pursuit of cures and treatments for infectious diseases, cancers, and monogenic disorders. AGT’s platform allows it to pursue therapies for large and orphan indications and complex diseases. The company has developed individual, intellectual property protected gene therapeutics that are breakthroughs in medicine. AGT’s pipeline includes Phenylketonuria (PKU), one of the most common monogenic rare diseases with an annual incidence in the United States of approximately 1 case among 13,500 live births.
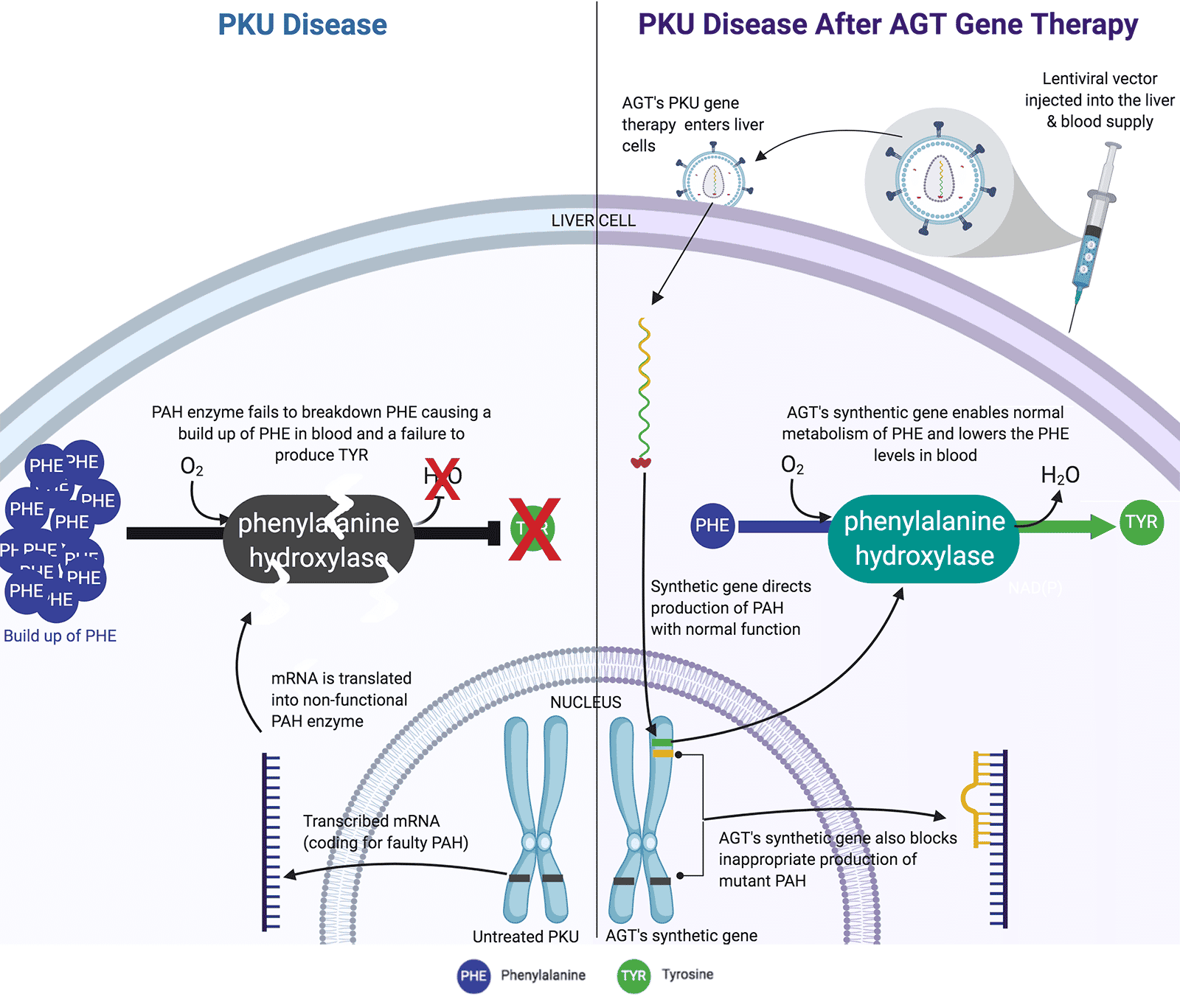
Source: AGT
PKU is caused by loss of function mutations in the gene Pah that encodes the enzyme phenylalanine hydroxylase (PAH). The loss of PAH function leads to excess accumulation of the amino acid phenylalanine (Phe), which reaches toxic levels in blood without strict dietary control. AGT is targeting PKU with a therapeutic strategy based on proprietary lentivirus vectors for modifying the liver to restore normal PAH activity and reduce Phe levels. The general nature and efficiency of lentivirus vectors plus unique features of our proprietary modifications are particularly suitable for permanent correction of PKU after a single therapeutic dose. The estimated North American and European markets for PKU treatments including medical food and supplements, exceeding $1 billion annually, could be replaced with AGT’s lentivirus vector gene therapy.