How to Design a Gene Therapy to Target Cancer


Contributing Author Luke Williams, Sales and Marketing
The cure for cancer is the holy grail of the medical sciences, so it may surprise you to learn that there are already two types of gene therapies that treat cancer. These cancer gene therapies are being used on multiple forms of blood cancer, and American Gene Technologies believes that there are many more cancer types which this unique therapeutic modality can address. Here, we’ll show you what cancer is, how new advances in genetic medicine address cancer in novel ways, and what challenges lie ahead as we design new genetic technologies to address the many forms of cancer that have recently fallen into therapeutic range.
What is cancer, exactly?
Cancer is a disease of uncontrolled cell growth, where unregulated cells spread to other parts of the body. Under normal circumstances, cells are tightly regulated to ensure that they only perform their intended function, and nothing else. You have ~200 cell types in your body, and each reads a specific set of genes to perform a specific function for your body. However, when the regulatory processes go off the rails, the cells can develop abnormalities.
Ordinarily, these abnormal cells are recognized and destroyed by the immune system. But depending on the abnormal cells’ characteristics, it may divide quickly, migrate within the body, or “convince” the immune system to leave it alone. Malignant tumors can be lethal, and may expand aggressively, so developing ready-to-use therapeutics that can differentiate cancerous cells from healthy bystanders is key to an effective anticancer therapy.
Traditional cancer therapeutics
Cancer can manifest in many ways, and modern medicine rises to meet it with an equally diverse set of treatment options. Doctors have a comprehensive toolset to use against the various forms of cancer, including:
- Radiation
- Hormone therapy
- Hyperthermia
- Over 100 chemotherapeutics
- Surgery
- And more
These treatment options are always being reevaluated against new therapeutic options to refine the toolset and achieve maximum effectiveness for each type of cancer. Continuous improvement saves lives and encourages innovation, making cancer research one of the most competitive fields in biotechnology and pharmaceuticals.
New creative angles that threaten to upend the standard of care represent hope for millions and a more efficient healthcare system for all. For example, oncolytic virotherapy is the practice of purposefully infecting a patient with a virus that preferentially replicates within cancer cells, which could theoretically provide a very powerful anti-tumor effect. These experimental approaches are typically used as last-line treatments to save patients who have failed to respond to other interventions, and as those therapies are refined, their use in wider patient populations or even first-line-treatment status can be adjusted to maximize the benefit to cancer patients. To predict the path ahead for new creative therapies, we’ll review the recent progress in oncology and explain how these newer therapeutics work.
Use the immune system to fight cancer - monoclonal antibodies
There are hundreds of distinct types of cancer, and depending on the type of cell the cancer arose from, it’s possible that the cancer cells may have characteristic markers on their cell surface that can be targeted using antibodies. The term “monoclonal” has a deeper immunologic definition, but to keep it simple, think of it as a set of antibodies that all recognize the same molecular pattern, and only that pattern. Figure 1 shows how monoclonal antibodies can be used to recognize cancer cells.
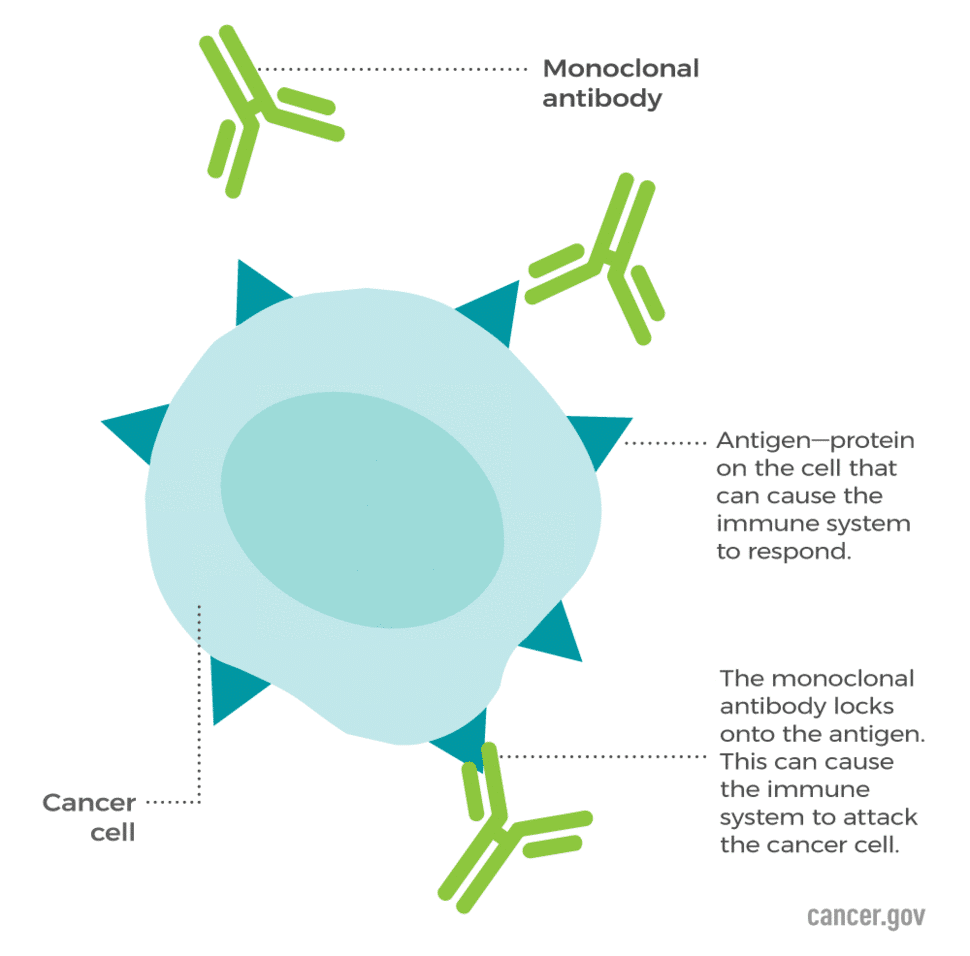
Figure 1: Antibodies can be designed to fit specific patterns on the surface of cancer cells known as cancer antigens. This marks the cells for immune recognition. Photo credit to the National Cancer Institute (NCI)
Antibodies are a natural weapon of the immune system, and under normal circumstances, they do not target the body’s own tissues. However, researchers can create custom antibodies which recognize molecular patterns that are commonly found on cancer cells, even if that pattern is normally seen as “self” by the immune system. By choosing the right molecular patterns, scientists can take advantage of the immune system’s “smart targeting system” to kill cancer cells while minimizing collateral damage. When the antibodies bind to cancer cells which bear the targeted pattern, they mediate immune responses which kill the cancer cells.
Tumors which have developed a wide array of abnormalities may not be good targets for monoclonal antibodies since they are such narrowly targeted tools, but if all of the cancerous cells happen to share a common pattern, monoclonal antibodies are an excellent way to selectively remove cancerous cells.
Monoclonal antibodies can also be used creatively to stick to receptors that inform the behavior of immune cells, selectively blocking the passage of certain information. These engineered antibodies are known as the immune checkpoint inhibitors. Checkpoint inhibitor therapy is used to block the “off switch” on tumor-targeting T cells so that these cells can keep attacking the tumor without being shut down.
Modify the immune system to fight blood cancer: CAR-T
Building on the theory behind monoclonal antibodies, chimeric antigen receptor T cell (CAR-T) is another form of anticancer therapy which augments the immune system’s weaponry.
CAR-T therapies take the precise pattern-recognition capability of a monoclonal antibody and combine it with the natural hunter-killer abilities of a T cell.
At a basic level, researchers can rework monoclonal antibodies to be compatible with the inner workings of a T cell, creating what is known as a Chimeric Antigen Receptor (CAR). Next, they can write the genetic code that translates into the CAR and deliver that CAR gene to T cells so they can make their own CAR molecules. After the gene therapy, these CAR-T cells are hard-wired to track down a specific molecular pattern, and are infused into the patient to find and kill cells bearing cancer antigens.
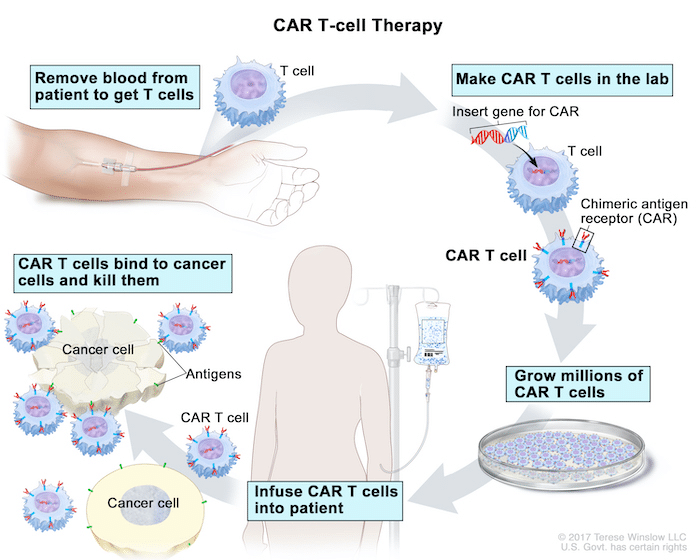
Figure 2: CAR-T cells are made by taking the patient’s own T cells and retrofitting them with new receptors that recognize cancer antigens.
CAR-T is one of the best examples of how gene therapy can be used as a way to enhance natural features of the immune system. Similarly, modifying T cells can also help researchers target viruses like HIV. Of the 6 CAR-T therapeutics that the FDA has approved for use (as of November 2022) you’ll notice that there are only two targets:
| Brand name | Target Antigen | Cancer Type |
|---|---|---|
| Kymriah | CD19 | B-cell acute lymphoblastic leukemia (ALL) B-cell non-Hodgkin lymphoma (NHL) |
| Yescarta | CD19 | B-cell non-Hodgkin lymphoma (NHL) Follicular lymphoma |
| CD19 | Mantle cell lymphoma (MCL) B-cell acute lymphoblastic leukemia (ALL) | |
| Breyanzi | CD19 | B-cell non-Hodgkin lymphoma (NHL) |
| Abecma | BCMA | Multiple Myeloma |
| Carvykti | BCMA | Multiple Myeloma |
Table 1: Adapted from cancer.gov’s page on CAR-T, this table shows the 6 CAR-T therapies approved for use in the US. Each CAR-T has a specific pattern that the CAR is designed to match, its target antigen. The specific form of cancer that the CAR-T is meant for is determined by the target antigen and other aspects of the therapy.
CD19 is a protein produced by B cells which shows up on the cell surface, making it a good marker for CAR-T therapies to target. Anti-CD19 CAR-Ts can effectively wipe out cancers that arise from abnormal B cells. While there will be collateral damage, it is constricted to the cells which express CD19. CD269, also known as B cell maturation antigen (BCMA), is another target for CAR-T therapeutics, and its expression pattern gives doctors the ability to target a subpopulation of cells in the B cell lineage for multiple myeloma.
Using the immune system to find and kill cancer cells is an exciting therapeutic avenue for many reasons:
- Remarkable specificity; you efficiently target and kill cells
- Living treatment – anti-cancer immune cells live with the patient long-term
- The immune system has natural anti-cancer abilities that we can amplify
For these reasons, the field of immuno-oncology is rapidly expanding, and American Gene Technologies is actively advancing the field in new ways using a little-known type of immune cell: the gamma-delta T cell.
Gamma-Delta (γδ) T cells for solid tumors - ImmunoTox
You might have heard people say “you get cancer 100 times a day” – and there’s an element of truth to that. The human body is made of trillions of cells, and if any one of those cells undergoes some type of transformation, a cancerous cell can arise. Between chemical exposure, radiation in the form of sunlight, or even just unavoidable errors in cells, cancer is a natural part of the human body. Accordingly, the body has onboard countermeasures.
The immune system is not just for defending against foreign entities, it also has antitumor capabilities. Classic alpha-beta (αβ) T cells are randomly generated, then pruned so that the cells which react to your own body are killed, leaving a mixed bag of T cells that can recognize anything foreign. In contrast, gamma-delta (γδ) T cells are randomly generated, but only the γδ T cells which recognize pieces of an inherited ledger of “danger signals” are allowed to mature. This means that the γδ T cells which circulate through the blood are specialized hunter-killers of some of the most notorious enemies across human history, whether they be foreign or domestic.
The controlled use of γδ T cells has several advantages over monoclonal antibodies and CAR-T:
- In certain types of cancer, it is unlikely that all cancer cells will bear the same cancer antigen which CARs or monoclonal antibodies can recognize. This makes the broad cancer recognition capabilities of γδ T cells more applicable to those types of cancer.
- Stimulating the natural anti-tumor capabilities of γδ T cells may allow researchers to eradicate tumors without introducing genetically modified T cells to the body.
- The γδ T cell response is a temporary phenomenon, so amplifying the power of the γδ response can lead to immune attack on the tumor which later resolves.
So how do we get the γδ T cells to attack? The Immunotox program argues that if we can inject a tumor with a gene therapy that destabilizes the tumor cells’ metabolism, we can cause the tumor cells to accumulate chemical “danger signals.” High levels of the danger signal excite the γδ T cells and activate an anti-tumor response. In theory, once activated by the danger signals, γδ T cells should circulate the body finding other abnormal cells, giving us the ability to hunt metastatic tumors all over the body, whether they received the gene therapy or not. At the end of the anti-tumor response, the tumor cells that received the gene therapy would be dead, the other tumor cells would have been hunted down, and the excited γδ T cells would settle back down.
Conclusion: How to design an anti-cancer gene therapy
If you want to cure cancer, you’ll need to target and kill cancer cells without causing too much collateral damage to the patient. Since cancer is an umbrella term for multiple distinct diseases which naturally arise from the body’s own cells, tumor targeting is complicated. The cancer cells in a tumor can “look” close enough to their healthy counterparts to avoid detection, but they can also have unique features relative to the other cancer cells in the tumor. Accurately identifying each and every cancer cell can be an incredibly difficult task.
Researchers have designed monoclonal antibodies and CAR-T therapies which can effectively target certain markers on the surface of cells, which allows them to direct the immune system’s weapons to a very narrow band of targets. This works especially well on cells of the B cell lineage, which have been targeted by all 6 of the FDA-approved CAR-T treatments, but not all cancer types arise from cells with such exploitable markers on their surfaces.
To target other tumor types, you’ll need to find another targeting system; one that can identify cancer cells based on a variety of criteria, no matter what cell type the tumor arose from. That’s a difficult task, so it helps to take a look at what nature has provided. By building on the immune system’s pre-existing anti-tumor capabilities, we can produce complex therapeutics with all the precision of the natural immune system. Sometimes, all the body needs is a little push, and with the right intervention, it may be possible to set off a chain reaction that eradicates tumors and their metastases.
There are multiple feasible strategies to deal with the various forms of cancer at different points of disease progression. Each therapeutic option is a tool that doctors can use alone or in combination with others, so it is the job of the scientist to create the best tools. For us at American Gene Technologies, the goal is clear: turn up the volume on the γδ T cell response and focus the full power of the immune system’s arsenal on tumors.





