American Gene™ CEO, Jeff Galvin’s Induction into the Montgomery County Business Hall of Fame 2022
Jeff Galvin has been inducted into the Montgomery County Business Hall of Fame (MCBHOF). The MCBHOF celebrates individuals who have made a substantial impact on the Montgomery County business community. Jeff Galvin was inducted on MCBHOF’s 10th annual event which was held on October 25, 2022. All net proceeds from the Montgomery County Business Hall of Fame benefit scholarship programs at The Universities at Shady Grove. You can make a donation to support these scholarship programs Click Here.
Dr. Conant was the first physician to diagnose HIV in San Francisco while running the inpatient dermatology service at the University of California San Francisco. On September 30, 2021, American Gene Technologies® appointed Dr. Marcus A. Conant, as special advisor to AGT™ CEO Jeff Galvin. Learn more about him in this video.


The Diagnostic Odyssey: A Rare Disease Parent Bootcamp
When you have a child, you want the best for that child in every way. For parents of children born with a genetic disorder, a correct diagnosis is the first step to receiving proper care. Unfortunately, there are many logistical and financial complications that make the diagnostic odyssey challenging for rare disease parents.
Amber Freed is a rare disease mom who has gone through incredible lengths to pursue a cure for her son battling SLC6A1. In the first of this Bootcamp series, Amber is joined by Brittany Stineman, the founder of SmashSMARD, and Holly Snyder, a genetic counselor from Illumina. In this session, Amber and Brittany share key lessons learned from years of rare disease research advocacy, and Holly highlights various genetic testing options available for parents and families seeking a diagnosis for their children.
Jeff Galvin, CEO of American Gene Technologies and Rich Bendis, CEO and President of BioHealth Innovation, Inc. have a fireside chat about the rapid evolution of the BioHealth Capital Region. Galvin and Bendis discuss the abundance of opportunities for growth within the region as it is home to a wide variety of research institutions, top scientists, and annual grants. Additionally, they discuss how BioHealth Innovation, Inc. cultivates success for companies within the competitive region.

Global Life Science and Pharmaceutical Headhunter Mantell Associates Network Interviews CEO Jeff Galvin
Mantell Associates Network interviews Jeff Galvin, CEO and Founder of American Gene Technologies® (AGT) for its global Podcast listeners. In this interview, Galvin shares the company's cell and gene therapy technology, toolset, and vision. Looking forward, host Alessandro Mantell and Jeff Galvin also get real about the industry and the forces at play that Galvin strategically manages so AGT makes progress independent of any obstacle(s). Listen now to learn what they uncover in this one-of-a-kind Podcast.
Jeff Galvin Presents at BIO International Convention Digital 2021
Jeff Galvin, CEO of American Gene Technologies, presents the latest news at the BIO Digital International Convention 2021. As the first patient in AGT’s Phase 1 clinical trial is infused with AGT103-T, an autologous cell therapy intended to cure HIV, the company expects to see safety data by the end of the summer, and an efficacy signal by the end of the year.

FIRST INFUSION News
AGT Treats First Participant with AGT103-T in Phase 1 Clinical Trial
AGT has reached a new milestone in its lead HIV program--the company treated its first participant with AGT103-T. AGT shared its news and media channels covered the story. These videos are a few of the stories and help explain this milestone.

ABC 7 News Live Segment by Justin Hinton

HIV Breakthrough at American Gene Technologies Gets Researchers Closer to the Cure

HIV Cure Program Clinical Trial Update - Spring 2021
Testing a Gene Therapy to Cure HIV & Fundamentally Change Healthcare with Jeff Galvin of American Gene Technologies®
From Lab to Launch by Qualio
Jeff is the CEO and founder of American Gene Technologies®. He earned his BA degree in Economics from Harvard and has more than 30 years of business and entrepreneurial experience including founder or executive positions at various Silicon Valley startups. Following his startup experience, he retired to become an Angel Investor in real estate and high tech. He came out of retirement to found and fund AGT after meeting Roscoe Brady at NIH and the incredible projects he was working on in gene and cell therapy.
The Future of TechBio: One on One with Jeff Galvin of American Gene Technologies
Biotech Bros
The Great Factor
By Muhammad Awan
Advancing a Cell Therapy with the Potential to Cure HIV
The Bio Report
AGT Covid-19 Testing Lab
AGT Opens RT-PCR Same-Day Testing Lab to Help Employees and Local Community
In the latest sign of the company’s commitment to its employees and the region, AGT recently announced the creation of a Clinical Laboratory Improvement Amendments (CLIA)-certified lab that is actively providing RT-PCR testing for COVID-19. AGT’s new COVID-19 testing capability provides less expensive, more convenient, and highly accurate same-day COVID-19 to its own staff and the general public. AGT’s testing program was designed to help its employees and other local organizations’ staff return to and remain safe in the workplace.

Biotech CEO Jeff Galvin Explains Cell and Gene Therapy to Stony Brook Biomedical Engineering Society
Jeff Galvin, CEO of American Gene Technologies (AGT), gives an inspiring talk at the Stony Brook University Biomedical Engineering Society (BMES) detailing his journey from working closely with computers in Silicon Valley to leveraging the progression of gene and cell therapy for curing major diseases after meeting Roscoe Brady.
"CURING INCURABLE DISEASES WITH JEFF GALVIN" | Lab to Launch Podcast by Qualio
Podcast
Qualio's brief summary:
"What if incurable diseases like HIV, cancer, PKU, and epithelial solid tumors were completely curable? We're closer than you might think. Really close. In fact, Jeff Galvin and his team at American Gene Technologies® (AGT) have been advancing the research and application of viral vectors for several years with some incredibly exciting breakthroughs on the horizon (hint: curing HIV could be just a few years away). In case you didn't know already, viral vectors is a relatively new technology where you can crack open any virus, scoop out the malevolent code and replace it with code to attack the root drivers of what are today incurable diseases. Essentially this creates a "stealth bomber" benevolent virus in your body. The future of this treatment could "send radiation and chemotherapy the way of bloodletting and leeches" as Jeff says in this podcast. Jeff has contagious energy for gene technologies and a grand vision of the future of curing the incurable."

How the Life Sciences Industry is Changing the World - "This Is USG" Video Podcast | Episode 15
Published by The Universities at Shady Grove
In this episode, Dr. Anne Khademian is joined by Jeff Galvin, CEO of American Gene Technologies, for a conversation about the life sciences industry and how it is changing the world with life-saving cures, treatments and technologies to improve health and the human condition.
AGT Named "Breakthrough Company of the Year" Award Winner by Biobuzz
This award recognizes the entrepreneurs and startup companies that have achieved strong commercialization momentum behind their innovative technology. Nominees were selected from companies that showed the most promise, the most momentum, and the most commitment to bringing their technology to the patients who need them.


Bio Innovation Visionary Jeff Galvin Presents "Death to the Regulatory Barrier to Better Medicine"
Jeff Galvin, CEO of American Gene Technologies, was the keynote speaker at the Bio-Innovation Virtual Conference organized by the Maryland Tech Council. During the conference, he gave this recorded presentation live: "Death to the Regulatory Barrier to Better Medicine."
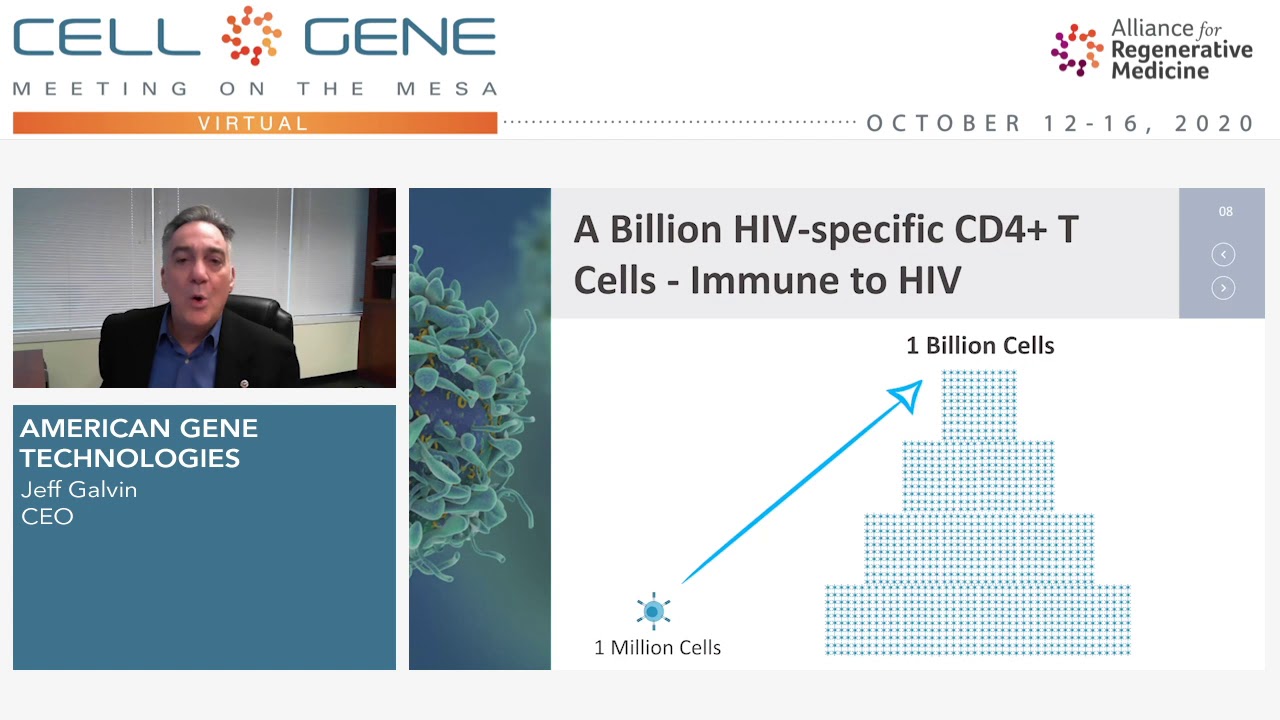
Jeff Galvin Presents at Cell and Gene Meeting on the Mesa
Recorded Presentation
CEO of American Gene Technologies (AGT) Jeff Galvin presents at the 2020 Alliance of Regenerative Medicine's Cell and Gene Meeting on the Mesa. In this presentation, he shares progress on AGT's upcoming Phase 1 clinical trial of its HIV cure program and its Immuno-Oncology approach to killing solid tumors.

iHeart Radio Recognizes Jeff Galvin as a “CEO You Should Know”
“CEOs You Should Know” is a weekly radio program sponsored by M&T Bank that profiles local companies and their leaders who drive the Greater Washington, D.C. regional economy. As a leader in DC’s business community, iHeart radio invited CEO of American Gene Technologies Jeff Galvin on-air.
C. David Pauza, Ph.D, Former CSO of AGT, Presents at the
Advanced Therapies Congress & Expo 2020

At the Intersection of Genetic Medicine and Immunotherapy: Clinical Experience with a Cell and Gene Therapy for HIV Disease.
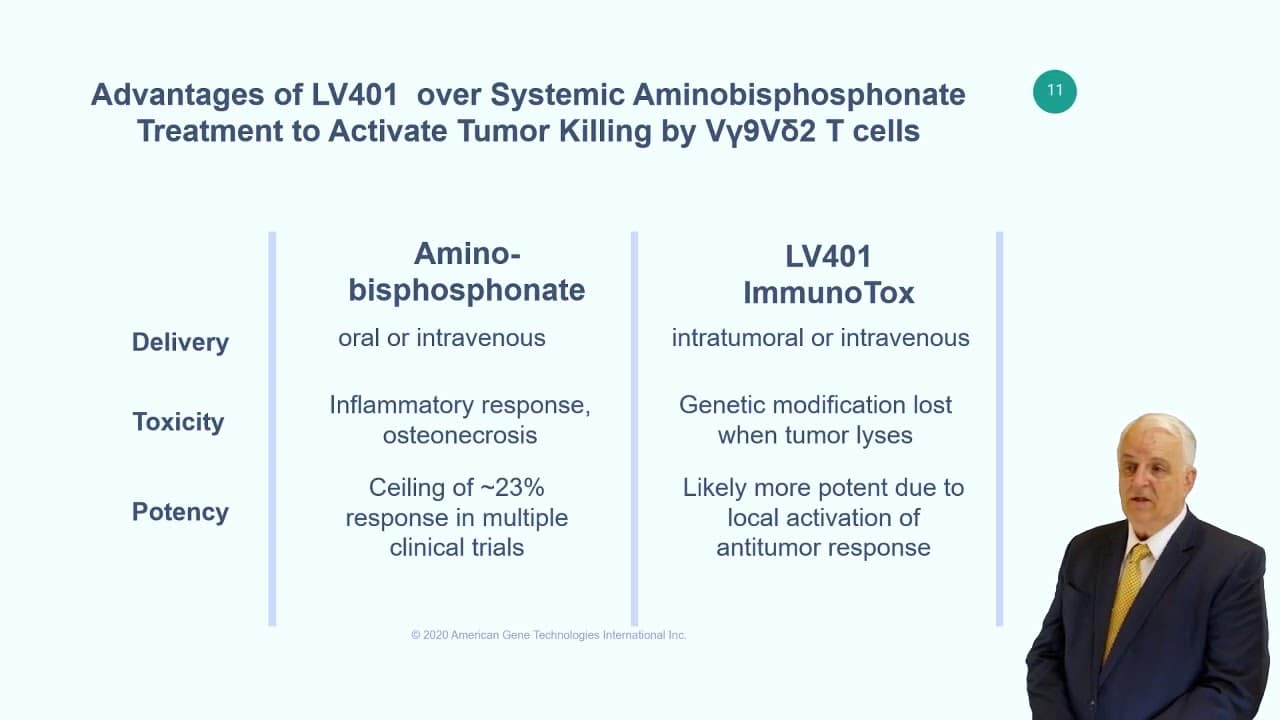
ImmunoTox: Genetic Manipulation of Solid Tumors to Activate the Natural Gamma Delta (γδ) T Cell Response
News Coverage
By ABC 7 Washington, D.C., CBS Baltimore, and DVM-270
When AGT announced its FDA approval to begin a Phase 1 clinical trial for its lead HIV program, media channels covered the story. These videos are a few of the stories that were shared about AGT's news. This event is a historic event for AGT as it marks the company's first clinical trial.
ABC 7 News WJLA: “Montgomery Co. biotech company receives FDA approval for human trials of HIV Cure program”

DVM-270 News: “Rockville lab to begin trials in attempt to cure HIV”

CBS News Baltimore WJZ: “FDA Clears Maryland Company’s Potential HIV Cure For Human Trials”

VIRTUAL PRESS CONFERENCE: FDA CLEARS AGT TO BEGIN PHASE 1 CLINICAL TRIAL OF HIV CURE PROGRAM
AGT was excited to announce its clearance by the FDA to begin a Phase I Trial for an HIV cure. The company hosted a virtual press conference to answer questions from reporters. Here is a video recording of the press conference.
Cell & Gene
Therapy Explained
An AGT Video Series
Companies like AGT are doing groundbreaking research and developing innovative ways to combat diseases. This series of videos will take an inside look at how gene and cell therapy technologies work, what it is like to be part of an innovative gene and cell therapy company, and what these technologies mean for the future of medicine.


2020 VIRTUAL PDA ANNUAL MEETING: Plenary Presentation on Gene and Cell Therapeutic Technologies Leading to Developing Better Pharmaceuticals at Lower Costs.
In this Plenary Talk at the 2020 PDA Virtual Annual Conference, CEO of AGT, Jeff Galvin explains how gene and cell therapeutic technologies are leading to more effective treatments while greatly reducing the costs of developing therapeutics.
CELL AND GENE THERAPY: DISCOVERING THE CLINICAL & COMMERCIAL POTENTIAL - Jeff Galvin Webinar
Jeff Galvin Presents "Discovering The Clinical & Commercial Potential of Cell and Gene Therapy" at the Databridge (DBMR) Conference Series on Cell and Gene Therapy on July 31st, 2020.


THE NEW ECONOMICS AND EFFICIENCIES OF GENE THERAPY - Jeff Galvin Presents at Biopharma Connections
In this BioPharma Connections webinar, CEO of AGT, Jeffrey Galvin explains how biotechnology is moving forward at an incredible rate. Gene and cell therapy is moving closer to an HIV Cure, and has been approved for a human trial since the recording of this webinar.
BIONEEX INTERVIEW WITH CEO OF AMERICAN GENE TECHNOLOGIES, JEFF GALVIN
BioNeex, a Biopharmaceutical R&D exchange platform for compounds licensing and co-development partnerships, created an interview series of innovative CEOs and executives to provide value to its customers and the biotechnology industry. Founder Dr. Smbat Rafayelyan interviewed AGT CEO Jeff Galvin as part of its series.


HIV IMMUNITY
Dr. Hardy Shares the Possibilities of Gene Therapy
Contrary to antiretroviral drugs, gene therapy could be used to give the human body immunity to HIV. Dr. David Hardy, a member of American Gene Technologies (AGT)’s HIV Scientific Advisory Board, tells his story of his lifelong commitment to mitigate the effects of the HIV virus.
GENE THERAPY TECHNOLOGY – REPROGRAMMING THE HUMAN COMPUTER
Jeff Galvin, CEO of American Gene Technologies (AGT), explains his ascent into the biotechnology drug development field after working closely with computers in Silicon Valley for a large part of his life.