American Gene Technologies® is a private biotechnology company supported by accredited investors. The company develops genetic medicines that address unmet medical needs in inherited or acquired diseases through the application of viral vector technology. American Gene Technologies is pioneering disease solutions to achieve permanent cures for human diseases.
The Future of Medicine
American Gene Technologies is focused on creating long-term value for patients, society, investors and donors by developing and testing cutting-edge gene therapies to cure some of humanity’s worst diseases. American Gene Technologies HIV cure clinical trial could not only lead to a blockbuster drug that brings relief to one of society's most elusive epidemics, but it could prove the efficiency and effectiveness of American Gene Technologies platform technologies to cure dozens of diseases ranging from viruses to cancers.

Barry H. Wells, MD
Investor Relations
U.S. Patents & FDA Designations
American Gene Technologies HIV, PKU and Cancer technologies are covered by an extensive patent portfolio.
DLA Piper Global Law Firm and Snell and Wilmer serve as American Gene patent counsels.
In addition, American Gene has been granted FDA Orphan Drug Designation for its treatment of PKU.
HIV Cure Program: Phase 1 Human Trial Accelerates
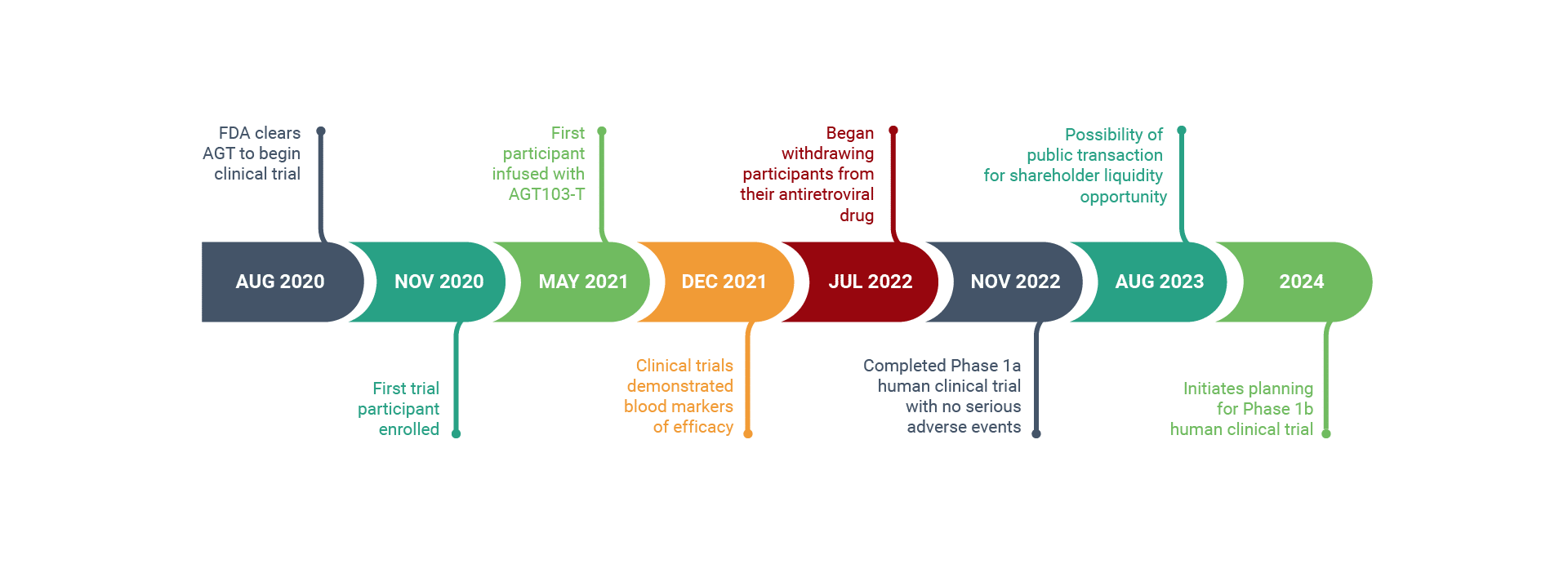
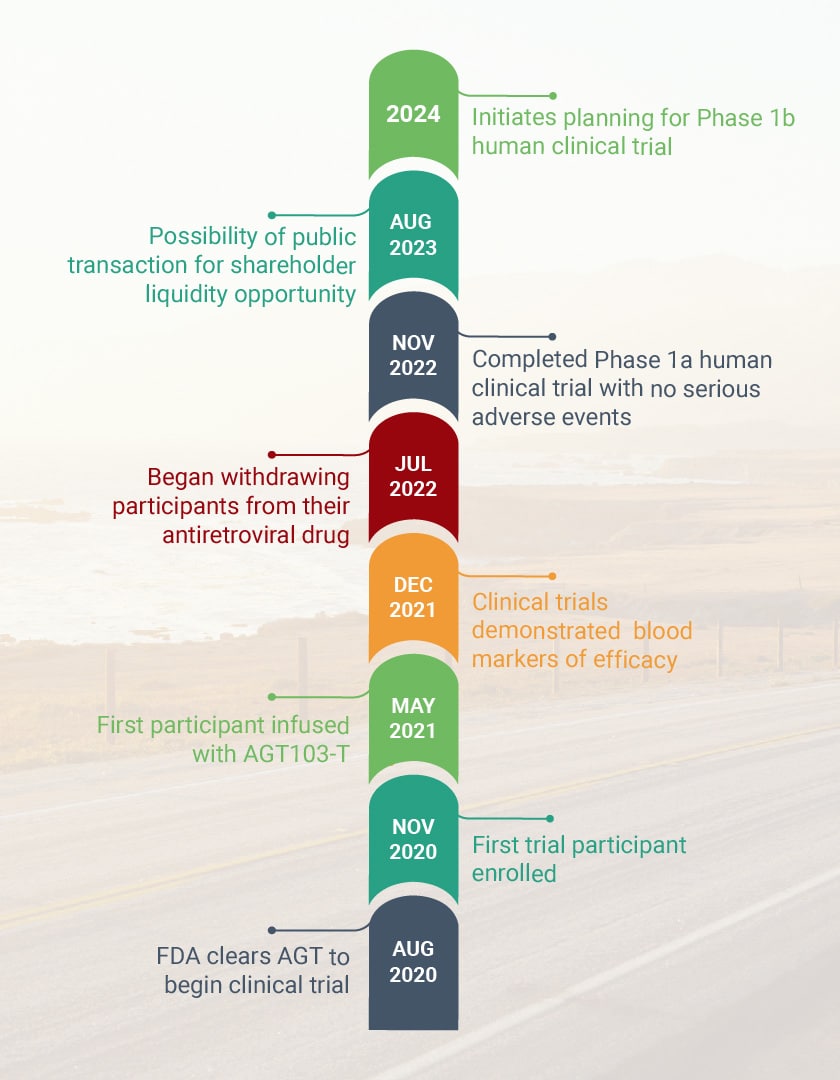
AGT ™successfully submitted its Investigational New Drug (IND) application to the Food & Drug Administration (FDA) to begin a Phase 1 clinical trial for its genetically modified autologous cell therapy for HIV.
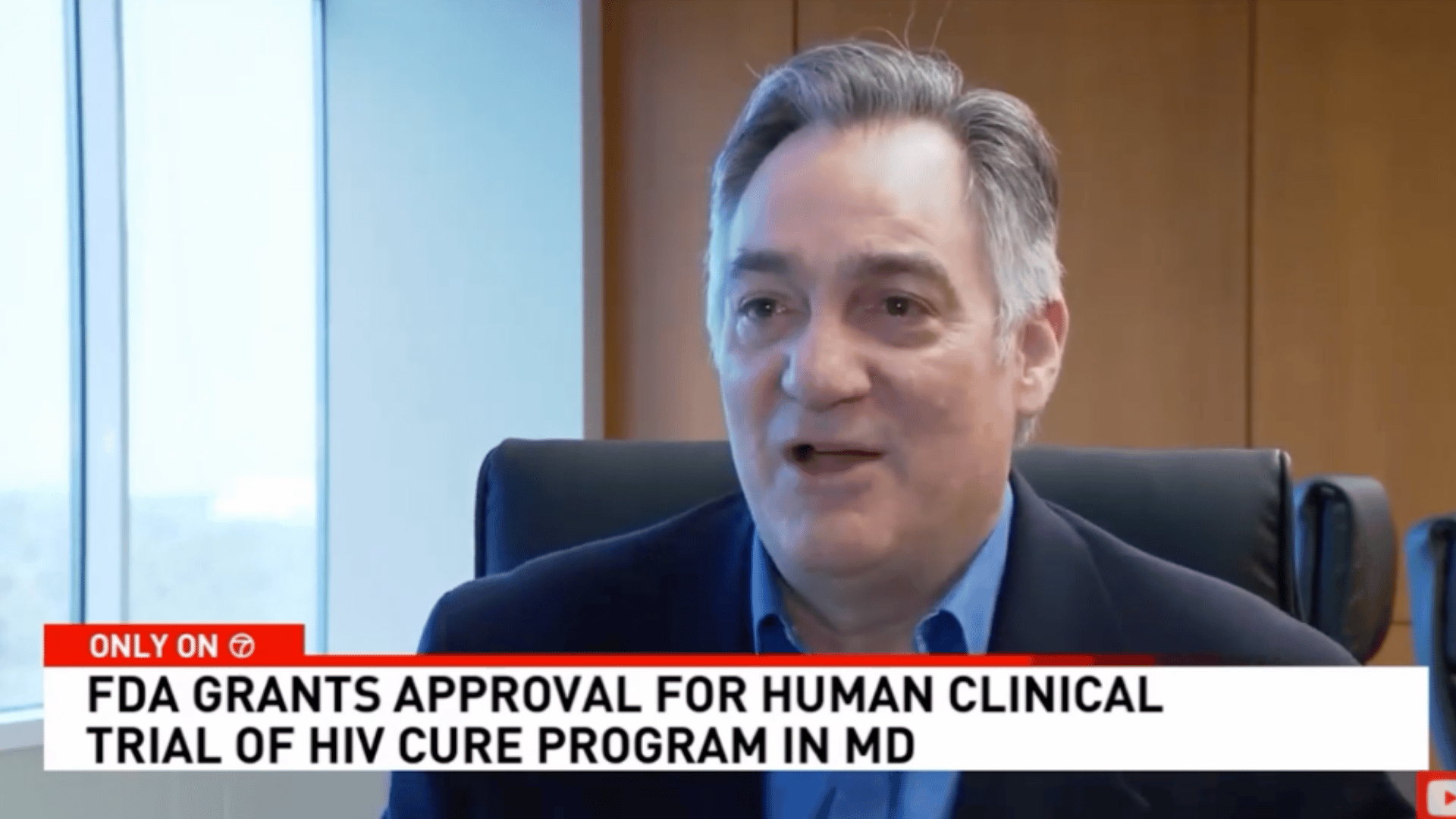
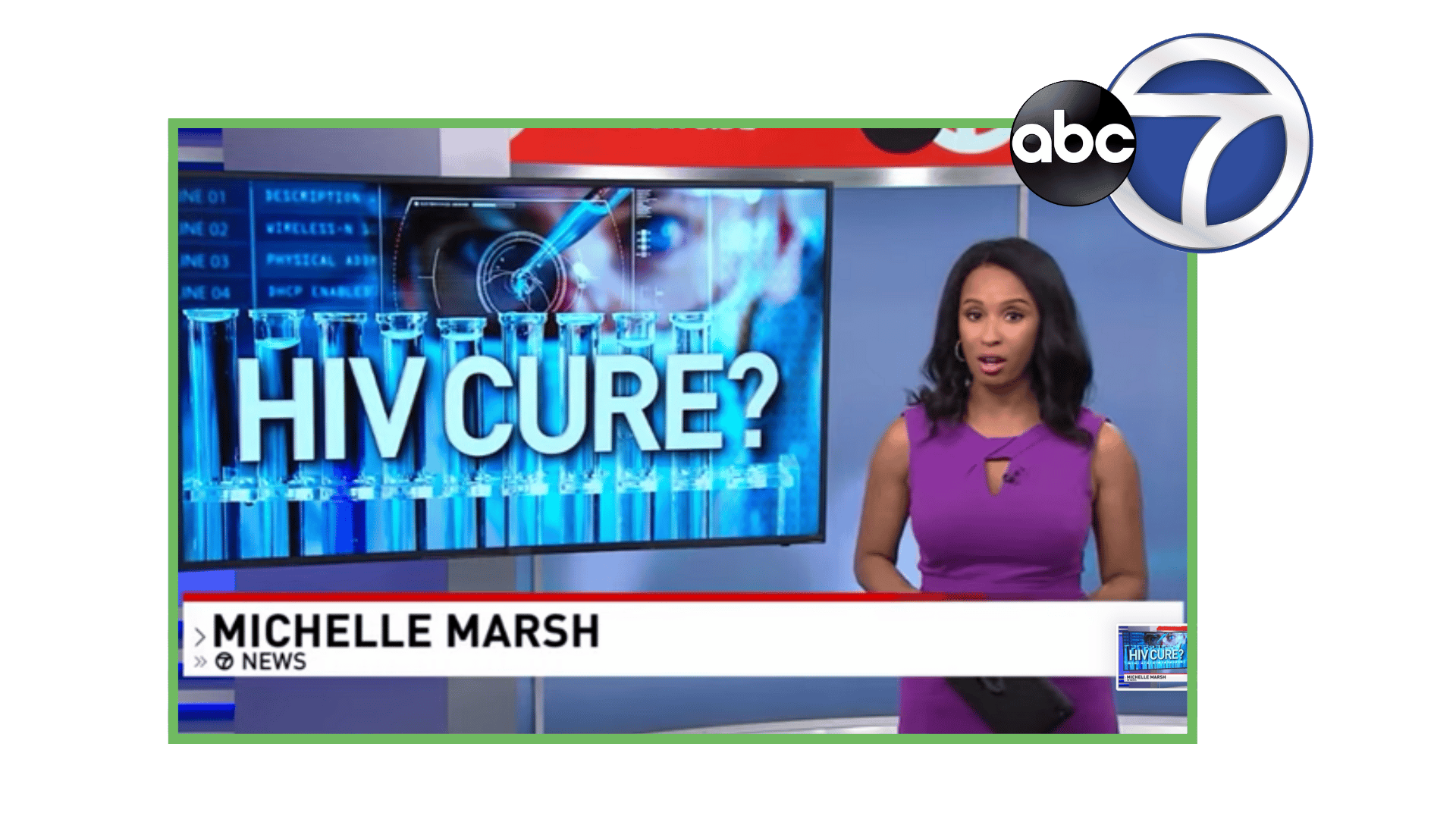
A Montgomery County-based biotechnology company has announced it received approval from the FDA to begin human trials for its HIV cure program. American Gene Technologies has been developing its program for 12 years. ABC7 News reports on the historic milestone.

American Gene Technologies announces the treatment of the first participant in its Phase I clinical trial to evaluate the safety of the cell and gene therapy product AGT103-T.

The Data and Safety Monitoring Board (DSMB) voted unanimously to continue American Gene Technologies HIV Cure Program without modification after safety analysis of the first participant’s data revealed no adverse effects from the treatment AGT103-T.
Third-Party Reports on
American Gene Technologies
American Gene Technologies hosted an analyst day to provide insight into the status of HIV therapy, including cure research, which provides a context for understanding the unique features and advantages of the company's AGT103-T cell and gene therapy for HIV. The event was hosted shortly before the IND was submitted.
American Gene Technologies is followed by the analyst listed above. Please note that any opinions, estimates or forecasts regarding American Gene Technologies performance made by the analyst are his alone and do not represent opinions, forecasts or predictions of American Gene Technologies or its management. American Gene Technologies does not, by its reference above or distribution, imply its endorsement of or concurrence with such information, conclusions or recommendations.
Our Leaders & Advisors
Jeff Galvin
Chairman, Founder & CEO
Tommy Thompson
Director
and Senior Advisor
Dr. Robert R. Redfield
Special Advisor
to the CEO
Dr. Marcus Conant
Special Advisor
to the CEO
We're happy to help
Contact Us

Primary IR Contact
Barry H. Wells, MD
Business Development,
Investor Relations
Sign-up For Our Newsletter
We send out quarterly newsletters that keep you up-to-date on all our projects, progress, and events.