HIV
Getting Close to a Potential Cure with Gene Therapy
American Gene Technologies® HIV Cure Program is preparing for a Phase 2 human trial.
The Future of Medicine
American Gene Technologies recognizes that gene therapy is so effective at curing diseases because it allows us to “edit” a cell’s operating system (its DNA) to correct the code or commands (genes). Gene therapy enables us to develop more specific and directed treatments and cures with fewer side effects because the drugs target diseased cells and avoid healthy tissue. American Gene™ is making great progress in our ongoing Phase 1 clinical trial where we are testing a gene therapy treatment that could potentially cure HIV.
HIV Science Advisory Board

John J. Rossi, PhD
City of Hope,
Duarte, California

Robert Clarke, PhD, DSc
Georgetown University
Medical School

Dean W. Felsher, MD, PhD
Stanford University
School of Medicine

Jerry Vockley, MD, PhD
University of Pittsburgh
School of Medicine

Fred Nyberg, PhD
Uppsala University,
Sweden
HIV Program Milestones
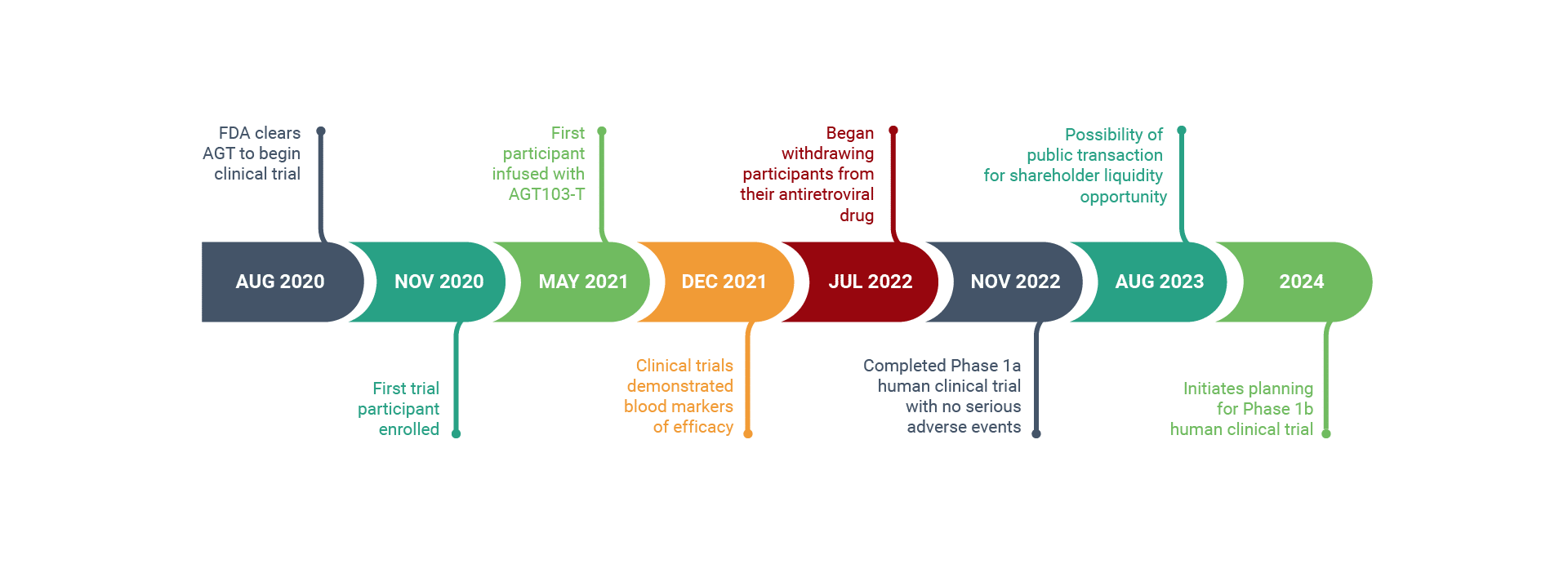
HIV Program Milestones
American Gene Technologies Plan for Immunotherapy of HIV
Our plan is simple. HIV depletes the HIV-specific CD4 T cell responsible for a potent immune response, necessitating lifelong antiretroviral therapy. If the depleted cells are replaced by durable T cells, natural immune control of HIV should be restored.
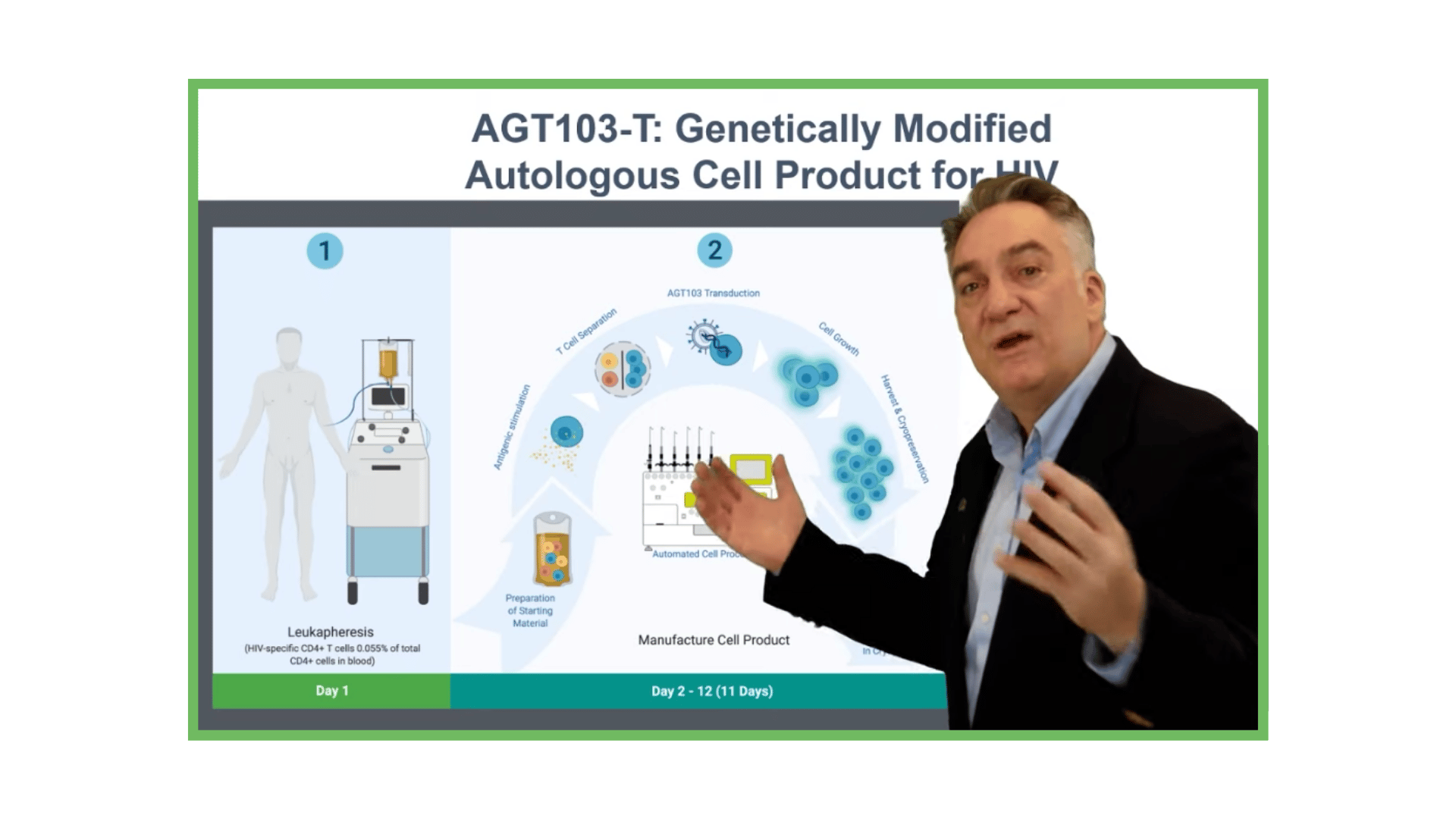
AGT103-T is a single dose autologous cell therapy delivering gene-therapy modified, HIV specific CD4 T cells to persons with HIV disease.
By repairing CD4 T cell immunity to HIV, AGT103-T promotes natural virus control and durable immunity from reinfection.
Rationale for AGT103-T
The manufacturing of specific T cells is used increasingly to make treatments for infectious diseases and cancer. In most cases, the processes are straightforward and cells are highly effective after being injected back into the person suffering from a disease.
A new industry is springing up around the need for cell manufacturing and is providing automation of highly efficient cell processing for T cell-based products. In most cases, specific cells are purified, grown to large numbers outside the body, then injected back in as a living cell therapy using the person’s own cells so there is no risk of rejection.
HIV infection depletes virus-specific CD4 T cells that are not reconstituted despite years of ART.
The critical CD4 T cell subset is the Gag-specific CD4 T cell population (around 10-4 frequency in persons with chronic, progressive HIV and virus suppressed by ART).
Elite controllers (EC) and long-term non-progressors (LTNP) are distinguished by much higher levels of Gag-specific CD4 T cells.
American Gene Technologies created a cell and gene therapy to raise Gag-specific CD4 T cell levels in patients with chronic, progressive disease and mimic the condition of EC and LTNP individuals.
Reconstituted Gag-Specific CD4 T cells may support durable immunity to HIV and reduce or eliminate the need for ART.
Patented Lentivirus Vector AGT103
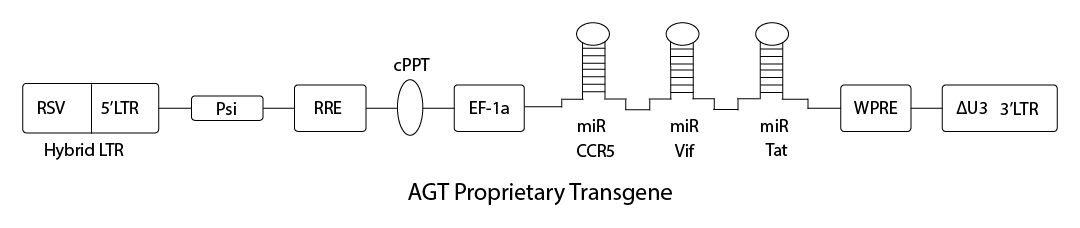
AGT103-T gives CD4 T cells the ability to survive attack by HIV and support effective antiviral immune response

Efficient transduction of primary CD4 T cells

Blocks HIV infection by CCR5- and CXCR4-tropic viruses

Prevents HIV replication in latently-infected reservoir cells
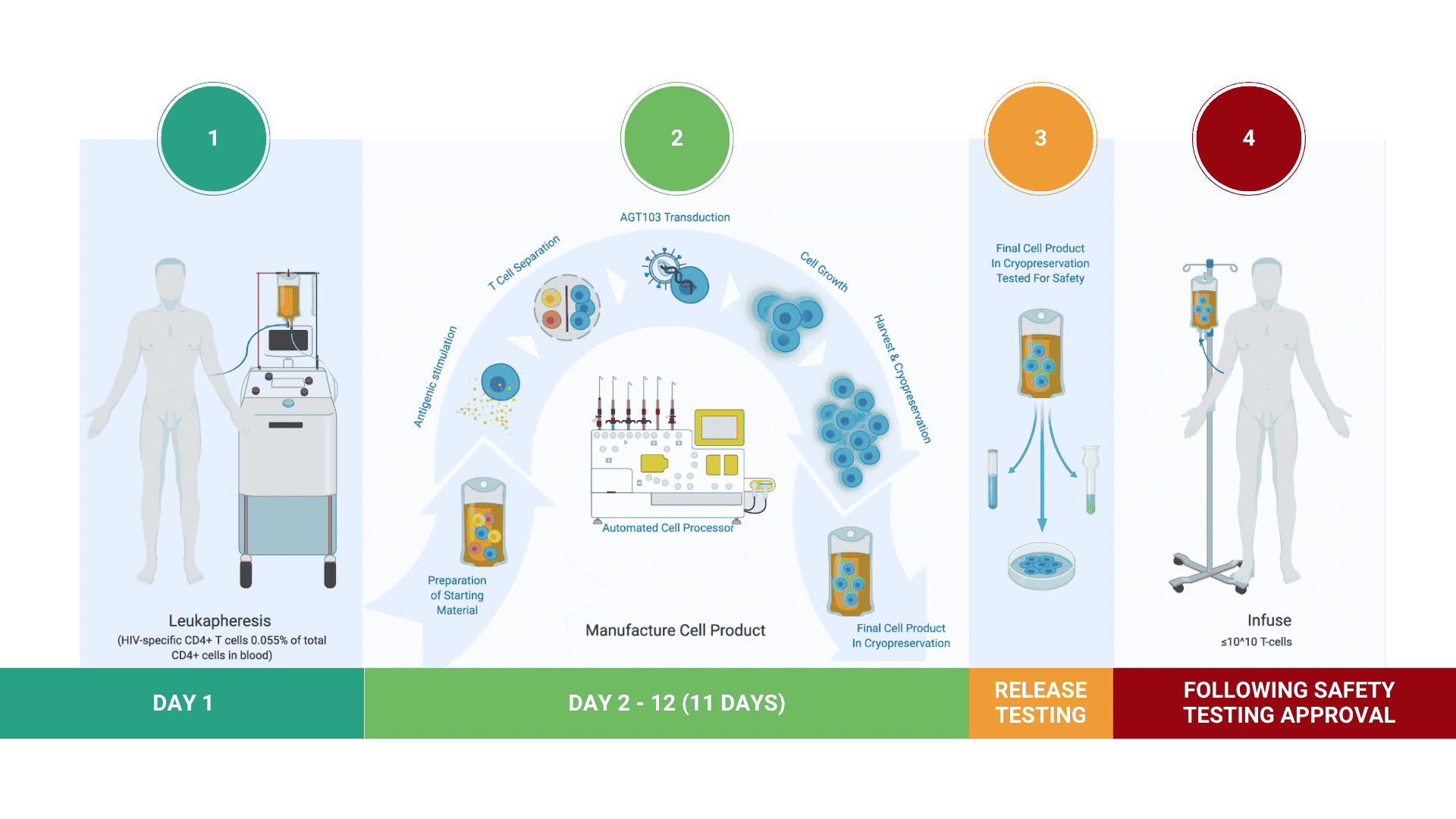
Manufacturing Cell Products for Treatment of HIV Disease
AGT has developed a highly efficient, cost-effective method for stimulating and selecting HIV-specific T cells, modifying them with lentivirus vector AGT-103T, and multiplying them for infusion back into the body as a single dose, autologous cell therapy.
Our Commitment to Developing New Treatments for HIV
Our company is developing genetic medicines for unmet medical needs. First among our priorities is a treatment for HIV that restores natural control of viremia and reduces dependence on antiretroviral therapy.
We believe natural virus control will be effective and will reduce the costs and side-effects inherent in life-long use of antiretroviral medication.