Treatment of Rare Diseases: What You Need to Know
Contributing writer: Gina Hagler
An emerging drug development model has the potential to reduce the cost of treatments for rare diseases.
What is a Rare Disease?
The definition of a rare disease varies by country, with the consensus that a rare disease or condition is one that affects far less than 1% of the population. Because each rare disease affects so few people, the traditional drug development model has proven to be inadequate when applied to them. Despite the provisions of the Orphan Drug Act, enacted in the United States in 1983 to stimulate investment in such treatments, the development cost for rare disease treatments has been prohibitive, making it virtually impossible for a drug manufacturer to recoup their investment during the time the treatment is under patent. As a result, the 350 million people worldwide who are affected by one of the approximately 7,000 rare diseases and patients are left without the likelihood of a viable and affordable treatment in their lifetime.
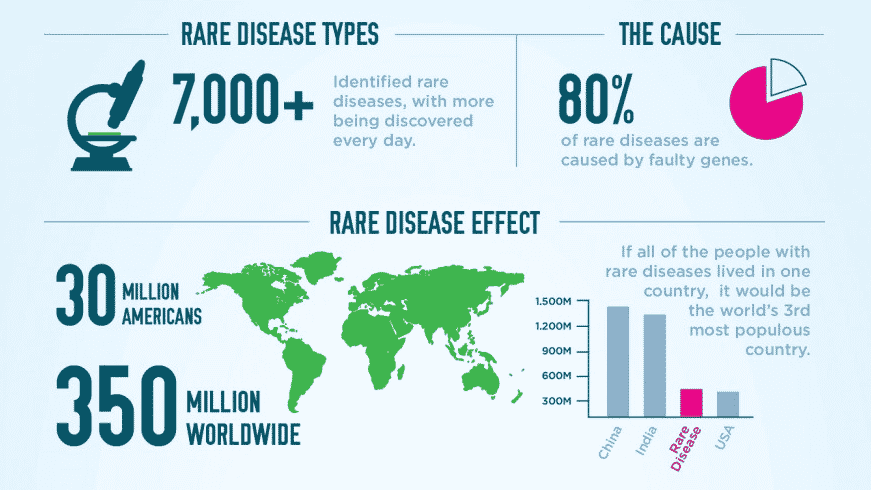
Photo Credit: World Rare Disease Day
How Much Does It Cost?
The current drug development process is time-consuming and complicated because it can take years of research to identify a likely treatment, formulate it for use by patients, and bring it through trials and onto the market. The process is also expensive. A recent JAMA study set the mean investment (inclusive of the cost of failed trials and research & development) at $1.4 billion. Treatments that are granted Orphan Drug Status by the FDA can cost considerably less to gain approval (read more). but If a pharmaceutical company is working on a drug without Orphan Drug status is to remain a viable business and continue its work on other drugs, it must recoup this money during the remaining life of the drug’s patent.
This is difficult at best as, despite the fact that there are thousands of rare diseases affecting millions of people, the total population constituting the market for a specific drug is typically not large enough to offset the costs incurred in bringing that drug to market. The result is a situation in which there is a financial disincentive for pharmaceutical companies to pursue treatment options for rare diseases. Because of this, reducing costs is vital.
How Can We Reduce These Costs?
Historically, due to the total cost, long development time, and short exclusivity period once the product is approved, there has been no viable way to reduce the costs associated with developing a new drug or recoup the cost after the drug is approved. Today, the growing field of biopharmaceuticals holds promise for the development of treatments for rare diseases, about 80% of which are genetic in nature. Gene editing and other tools of biotech can be brought into play as potential game-changers. Whether a conventional treatment or a biopharmaceutical treatment is being explored, the need for funding in some areas remains unchanged, i.e, the research and development effort, the formulation of an effective delivery system, and the design and implementation of trials that are populated with those affected by the disease.
The rapid progress made in developing such biopharmaceutical tools as AGT’s gene delivery platform in recent years offers an opportunity to reduce the financial investment in a new treatment by reducing the costs associated with these activities by working to:
1. Maximize the Return on R&D Expenditures
One way to lower the costs of treatments for rare diseases is to minimize the expenses associated with research and development. Some large biopharma companies attempt to spread R&D costs across a range of drugs by pursuing promising research in several areas and matching their findings with existing problems when the research indicates they have a potential treatment. This approach to drug development is not specifically done with the intention of finding a treatment for a particular rare disease. As a result, the company’s overall profitability is enhanced by dedicating its research to scientific discovery rather than the pursuit of a treatment for one specific condition.
For rare diseases, this is not the most expedient way to pursue a cure; those awaiting a treatment usually have a limited window in their lifespan during which treatment will make a meaningful difference. Organizations and labs that are dedicated solely to research for a specific rare disease must put their focus on one target through a limited number of possible pathways. This focuses their R&D dollars on one disease, making it more likely that a treatment will be developed in a relatively short amount of time. Since this essential work is costly, the only realistic way to maximize the return on this R&D is to achieve a possible treatment in the shortest amount of time, then move forward with a proven delivery system and remote trials.
2. Make Use of Orphan Drug Status When Possible
The Orphan Drug designation helps to decrease the cost of rare disease development through tax credits. The designation also provides exclusivity for seven years after approval. To read more about the benefits from the Orphan Drug designation and incentives, see “Benefits of FDA Orphan Drug Designation: What You Need to Know.”

3. Make Use of a Proven Delivery System
Many companies in the life science industry have chosen to specialize in one role. American Gene Technologies (AGT) has claimed its place as a company that delivers the solution to the patient with what Jeff Galvin, AGT founder and CEO, describes as “genetic tools and reusable components that can be mixed and matched to cure a myriad of diseases.”

In 2019, AGT received an FDA orphan drug designation for the treatment of phenylketonuria (PKU) using its proprietary, lentiviral vector-based technology. If successful, AGT’s lentiviral vector approach will provide a permanent cure for more than 16,000 people living in the U.S. alone. Galvin says, “We believe this orphan designation will enable AGT to formally shape and advance its PKU program.”
The first clinical trial utilizing AGT’s technology will be for a functional cure for HIV/AIDS, a disease that is far from rare. However, the tools and know-how developed for its HIV program will benefit AGT’s work in PKU as well.
Both of these potential cures utilize the delivery system Galvin spoke about In a recent TEDx Talk given at Georgetown University. As Galvin stressed, utilizing this existing delivery system has made it possible to go from the R&D stage to clinical trials with very little additional time or money invested.

4. Make Remote Participation Possible
Given that rare diseases affect very few people around the world, the costs associated with the logistics of the trials that are required to prove the efficacy and safety of a treatment are significant factors contributing to the cost of the treatment. Harsha Rajasimha, Ph.D., founder and CEO of Jeeva Informatics Solutions Inc., stresses that "the top thing to know about treatments for rare diseases is that access depends on the patient's ability to travel and their zip code. With 95% of rare diseases still without any FDA approved therapies, thousands of treatments are still in clinical trials.
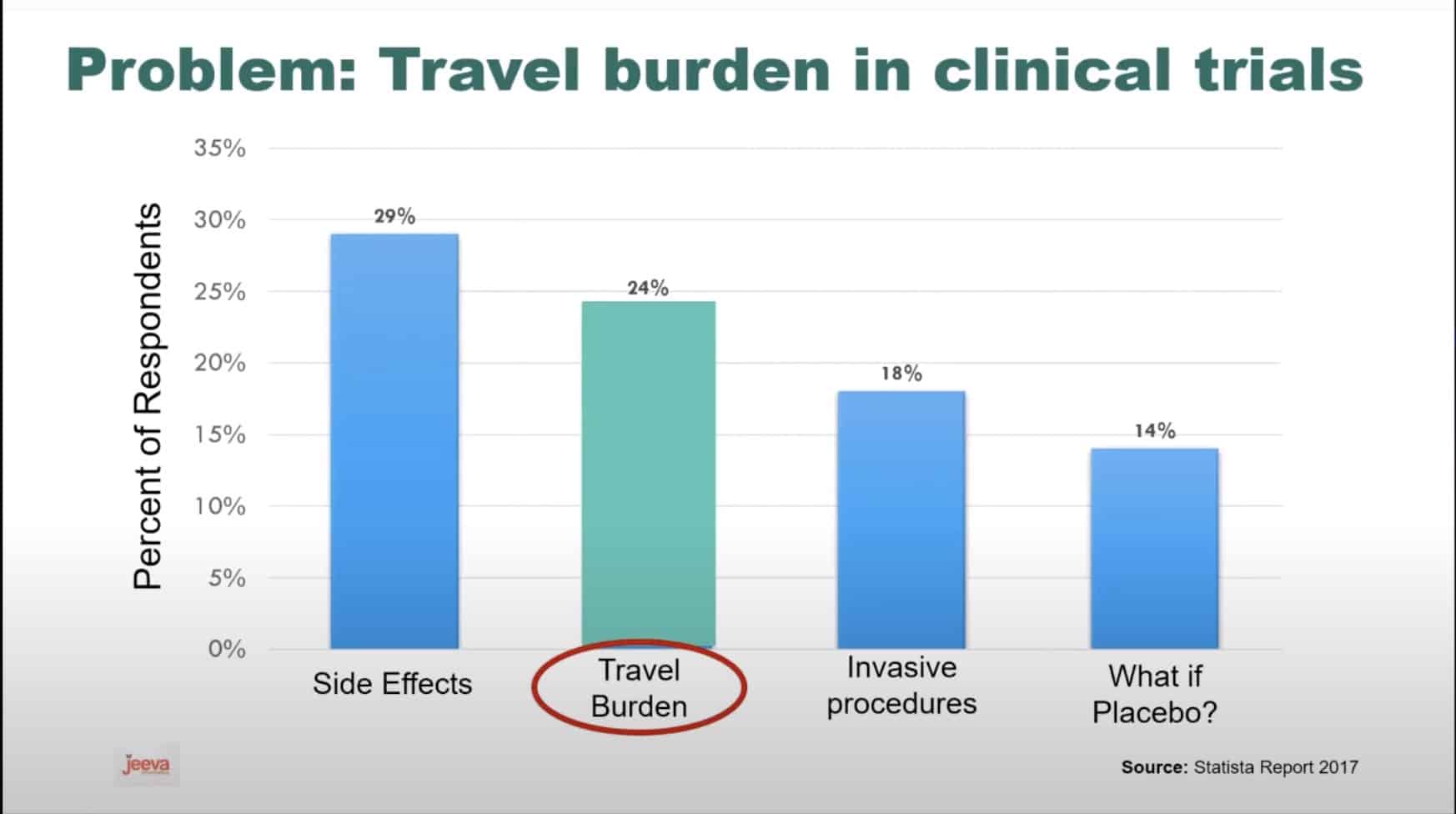
Almost all of these clinical trials are sponsored by biopharmaceutical companies in the USA and the EU. So, there is an inherent bias in who gets to participate and access these potentially life-saving therapies before they are approved years down the road. We are developing Saas [Software as a Service]solutions to reduce this travel burden for patients in clinical trials by 50% to 80%." The ability to participate remotely in a trial will not only make it easier for distant patients to participate in trials but it also reduces the logistical costs of these trials significantly.
Bottom Line: The Cost of Rare Disease Treatments will Decline
As the search for treatments for rare diseases continues, the advances made in gene and cell therapy bode well for these largely genetic disorders. By combining research with the benefits of Orphan Drug status, new delivery methods, and remote participation in trials, a new drug development model is evolving. This model makes use of efficiencies that did not exist until recently. Their part in bringing about a decline in the initial investment for drug development makes it possible for biopharma companies and labs to pursue treatments that would have been prohibitively expensive in the past. As Jeff Galvin wrote in a recent article on biopharmaceuticals, “Gene and cell therapies are making an impression on the entire medical community and even the public. The possibilities are endless. This is no longer science fiction. It is the future of medicine. And that future is bright.”