Gene Therapy: More than Monogenic Disease

Contributing Author John Vandermosten, Senior Biotechnology Analyst
Gene therapy is a diverse technology that can address a broad variety of diseases, conditions and disorders. About a year ago, the FDA approved its latest gene therapy product, Zolgensma, for spinal muscular atrophy (SMA), a neuromuscular disorder caused by a mutation in the SMN1 gene. Gene therapy is a natural fit for solving monogenic diseases such as SMA given the technology’s ability to insert missing sequences in a patient’s DNA. Gene therapy is particularly applicable to monogenic abnormalities as it is able to repair mutations in a single gene and has over 7,000 existing monogenic diseases to choose from.
Despite the obvious applications, developing a single drug in the monogenic disease space that must pass through the regulatory approval process is prohibitive for what in many cases are ultra-rare diseases. Only a few individuals have any single one of these afflictions, creating financial and resource hurdles to addressing them under the current approach. These limitations have not held back the advance of gene therapy, and the power of the technology has expanded the field beyond monogenic disease. Much of the groundbreaking work being done in gene therapy is focused on areas such a polygenic diseases and complex acquired disorders.
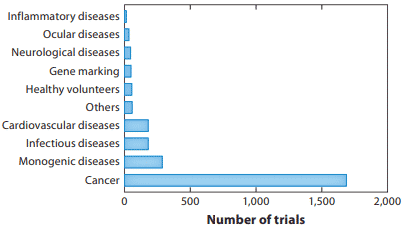
We count over 80 gene therapy candidates in clinical development1 in addition to the 20+ approved products around the world. Most gene therapy trials are being conducted in cancer, but cardiovascular, infectious and neurological diseases are other material pursuits.
Gene Therapy Indications, November 20172
Gene therapy can be deployed in many ways. Its abilities span from replacing a defective gene, either in vivo or ex vivo, to the modification of RNA to make a functional protein. The technology can be used to produce therapeutic cells, deliver genes that will stimulate the growth of new tissue and deliver bacterial or viral genes as a mechanism of vaccination. Approaches can either be integrative or non-integrative depending on whether the modification is desired to persist into daughter cells. There are also non-viral vector methods of delivering genes and nucleoprotein complexes including lipofection, electroporation and nucleofection.
We explore the diversity of gene therapy in this brief review and examine a cross section of three non-monogenic approaches that are being pursued with gene therapy. From multi-hundred million dollar approved cancer treatments to early stage infectious disease, we provide a selected profile of the groundbreaking work that is being done with this technology.
- Novartis – Kymriah: B-cell acute lymphoblastic leukemia; ex vivo CAR-T cell generation
- Inovio – VGX-3100: HPV related epithelial dysplasias; DNA plasmids delivered by electroporation
- AGT – AGT103-T: HIV functional cure; lentiviral transduction performed ex vivo
Kymriah
One of the best known programs that employs gene therapy is Novartis’ chimeric antigen receptor T (CAR-T) cell receptor therapy, which engineers T cells to express proteins with an affinity toward cancer cells. The so-called “living drug,” branded Kymriah and generically known as tisagenlecleucel, was originally developed by a group at the University of Pennsylvania led by Carl H. June. Genetically modified immune cells expressing either CARs or T cell receptors isolated from tumor-reactive T cells has shown success with the treatment of certain blood cancers. CARs enable the gene-modified cell to specifically target antigens expressed on the surface of tumor cells.
The approach extracts T cells from a patient through a blood draw, then sends it to a manufacturing site which modifies the cells using gene therapy. The approach uses a lentiviral vector to deliver the genetic instructions for the CAR T cell enabling it to target CD19 expressing tumor cells. After the modified cells are manufactured and expanded, they are infused back into the patient where the augmented T cells find and destroy the patient’s leukemia.3 Kymriah is forecast to generate over $430 million in sales in 2020 and break through $1 billion in sales by 2024.4
Kymriah was the first CAR-T therapy approved by the FDA on August 30, 2017 and was the first gene therapy-based treatment approved for use in the United States. It was followed shortly after by a similar approach in another CAR-T cancer drug called Yescarta (axicabtagene ciloleucel) for large B-cell lymphoma. The Kite Pharma-sponsored medicine also targets CD19 and was approved in October 2017 using a retrovirus as its gene editing vector. Both were later approved by the European Commission.
VGX-3100
Inovio has a novel approach to gene therapy through the use of its DNA medicines in a variety of infectious diseases, HPV related epithelial dysplasias and cancer. The platform can generate multiple antigens manufactured by the host’s cells to mobilize the attack of pathogens more thoroughly than single antigen approaches. In contrast to gene replacement or gene insertion, a standalone DNA plasmid is used. The plasmid contains a sequence to produce a target antigen once inside a cell.
Rather than employ a viral vector to deliver the DNA plasmids to cells, an electroporation device is used. The machine generates a brief electrical pulse to open pores in a cell, allowing the plasmids to enter. Once inside, the plasmids engage the cellular machinery to generate the target antigens, which activate the immune system’s antibody and T cell responses to eradicate the HPV virus.
VGX-3100 is built on this DNA medicine platform and is a Phase III candidate being developed for human papilloma virus (HPV)-related cancers and dysplasias. It encodes DNA for modified HPV-16 and HPV-18, E6 and E7 proteins to stimulate an effector T cell response. HPV-related dysplasias, including cervical, vulvar and anal dysplasia affect up to a million women per year and efforts to vaccinate for HPV have only been partially effective. This has increased the need for treatment and for VGX-3100. Interim data for the Phase III clinical trial in cervical dysplasia is expected by the end of the 2020.
AGT103-T
Another exciting and innovative approach to using gene therapy is being investigated by American Gene Technologies to advance an HIV cure. The company is using a lentiviral vector to deliver a gene sequence encoding inhibitory RNA targeting the CCR5 receptor on the surface of T cells and HIV proteins that have infected the T cell. Silencing the HIV proteins also prevents the cell from releasing HIV if it was infected at the time of transduction. The ex vivo autologous cell therapy removes T cells through a blood draw; the cells are then expanded and modified using the lentiviral vector. Modified cells are then infused into the patient where they are able to target HIV and essentially eradicate the virus.
The process is similar to that used in CAR-T therapy, which modifies autologous cells ex vivo to carry on therapeutic work in the patient. AGT103-T cleverly evolves past the shortcomings of other HIV approaches by expanding the specific HIV T cells that are essential to eradicating the virus and suppressing the viral proteins critical for HIV’s propagation. AGT103-T addresses the immunological defect in HIV and repairs the capacity of the immune system to present a normal response and control HIV naturally.
According to UNAIDS, there were about 38 million people globally with HIV/AIDS in 2019 and according to the CDC, 1.2 million in the U.S. in 2018. While the number of people dying from the disease has slowed since the advent of antiretroviral therapy, there were still almost 16,000 deaths in 2018 related to HIV. Beyond the heightened risk of death, HIV patients also must take an expensive and harsh regimen of medications for the rest of their lives further highlighting the need for a functional cure.
Summary
Gene therapy is a natural fit for addressing monogenic diseases; however, its application is much broader and there are numerous programs underway that address other classes of disease in innovative ways. In this brief review we examine three programs that are using gene therapy to cure infirmity and expand beyond the initial monogenic aspirations of the technology. One of the best recognized gene therapy approaches is Novartis’ Kymriah for blood cancer which uses gene therapy to modify a patient’s cells outside the body, then return them to identify and kill CD19 expressing cells. Yet another innovation is Inovio’s DNA medicine which uses the body’s cellular machinery to generate antigens which activate the immune system against viruses that are normally able to hide. A complex and intrepid approach by AGT to cure HIV combines ex vivo cell modification with a three part cargo designed to discourage HIV from penetrating its target and silencing its ability to replicate, allowing the trained T cells to eradicate the virus. These programs provide veritable evidence of the broad applicability of gene therapy to not only repair monogenic defects but also embrace complex approaches to eradicate cancers and modify cell activity.
1 Source: Evaluate Pharma as of July 2020.
2 Anguela, X., High, K. Entering the Modern Era of Gene Therapy. Annu. Rev. Med. 2019. 70:29.1–29.16
3 Kymriah is specifically indicated for the treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse.
4 Estimates sourced from Evaluate Pharma.





