Rare Diseases: Why Are They Difficult to Cure?

Contributing Author: Gina Hagler
Approximately 7,000 rare diseases affect 350-500 million people worldwide. The majority of these (80%) are monogenic, so-called because these genetic disorders are caused by a mutation in a single gene. While gene therapy holds great promise for the treatment of monogenic diseases in the future, efforts to find cures to date with traditional pharmaceuticals have resulted in treatments for only 5% of all rare diseases. Why has it been so difficult to find rare disease cures?
Difficulty in Diagnosis
Before the Human Genome Project (HCP) was completed in 2003, researchers did not have access to the complete genome of our species. Without this knowledge, it was impossible to arrive at an accurate diagnose of the cause of a genetic disease; the best that could be done was to attribute the symptoms to a type of disease based on its characteristics. Once that was done, there might be treatments that would help with the symptoms, but there would be no cure. With the use of genetic testing, it is now possible to identify the exact location of the defective gene. Even with the resources available to us today, it still takes time to make a diagnosis, as was the case with SLC-6A1 and Amber Freed’s son, Maxwell, which we wrote about here.
Small Patient Population
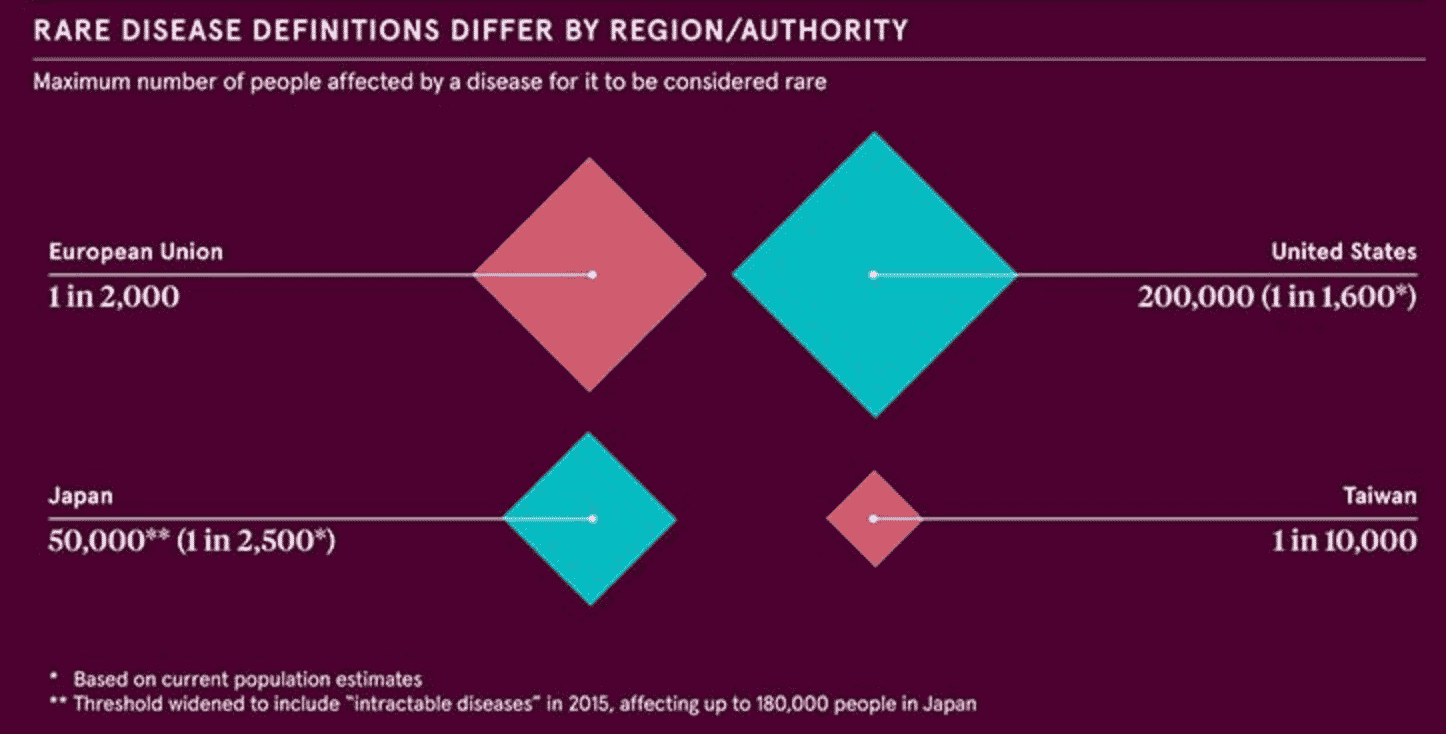
The exact definition of a rare disease varies by country, but no matter the definition, rare diseases affect 1% or less of the global population. Since there are many rare diseases within that 1%, the population for any one disease is very small. Because those affected are located around the world, they are unknown to one another or to those conducting rare disease research. The geographic spread, combined with the small number of people affected, has made it difficult to gain recognition as a population in need of a cure.
The National Organization for Rare Disorders (NORD) has created a site with resources that include information on more than 1,200 diseases and a listing of clinical trial and research studies by disorder. NORD is making it possible for those with rare disorders to work together to advocate for themselves because “Alone we are rare. Together we are strong.”Ⓡ The use of technology to make remote participation possible in research and clinical trials, as described in our article, Treatment of Rare Diseases: What You Need to Know, is also a potential game-changer.
Profitability
Any company sets out to earn a profit on the products it creates. The company needs to clear a profit overall if it is to remain viable. As we discussed in Treatment of Rare Diseases: What You Need to Know, the argument has long been that the cost of research and development for these rare conditions is prohibitive and the patient population small. There was no way for the pharmaceutical industry to profitably produce new treatments.

The financial benefits for pharmaceutical companies working on treatments for orphan diseases through the Orphan Drug Act are explained more fully in our article, Benefits of FDA Orphan Drug Designation: What You Need to Know. These benefits are making a difference in the profitability equation. As a result, firms like American Gene Technologies (AGT) are pursuing disorders like Phenylketonuria (PKU) with the use of an Orphan Drug Designation.
Show Me the Cures
1. Sickle Cell anemia affects about 100,000 people in the United States and millions worldwide. Until last year, the only possible cure was a stem cell transplant. However, since there is the risk of graft vs host reaction with a stem cell transplant, it wasn’t considered a viable cure. All of that changed in March 2019 when Janelle Stephenson, 28, received a genetic treatment as part of an NIH clinical trial. The replacement of the single defective gene, the beta-globin gene, made it possible for Stephenson’s cells to produce anti-sickling hemoglobin. Her cure is believed to be one-and-done: one in which there is no need for ongoing treatment.
2. Phenylketonuria (PKU) affects various ethnic groups and geographic regions worldwide. In the United States, it occurs in 1 in 10,000 to 15,000 newborns. All newborns are tested for PKU within 72 hours after birth with a simple heel prick to generate the small amount of blood needed for the simple test for PKU. It is essential to identify those infants at risk for PKU, so that they can be started on an effective treatment and eat a diet that limits foods containing phenylalanine during infancy. As they grow older, they must avoid foods that are high in protein. Failure to follow these guidelines results in intellectual disabilities caused by a buildup of the amino acid phenylalanine, which is toxic to the nervous system. There is currently no cure for this debilitating disease, although American Gene Technologies (AGT) is working toward a cure for PKU.
3. Cystic Fibrosis affects about 30,000 in the United States. and more than 70,000 people worldwide. Until recently, attempts to deliver a working copy of the gene necessary to a cure have not been effective. With advances in viral vectors, this is changing. In 2019, the Cystic Fibrosis Foundation announced $500 million in funding over the next six years for research into treatments for cystic fibrosis. These include gene therapy strategies.
4. Hemophilia A affects an estimated 400,000 males worldwide, with about 20,000 in the United States. There is currently no cure for this genetic disease. In August 2020, the U.S. Food and Drug Administration (FDA) rejected a gene therapy called Roctavian and asked BioMarin Pharmaceutical for more evidence that the treatment is durable enough to justify use in patients by proving a long-lasting effect on bleeding rates that will not wear off, resulting in a return to the current standard of care for patients.
5. Muscular Dystrophy (MD) The most common forms in children, Duchenne and Becker, affect approximately 1 in every 5,600 to 7,700 males ages 5 to 24. There is currently no known cure for this genetic disease. However, the use of CRISPR gene-editing technology may lead to a long-awaited cure or treatment options.
Bottom Line
The outlook for rare disease cures is excellent. The ability to make a diagnosis sooner rather than later gives those with the disease more time to identify and participate in promising research and trials. Information such as that on NORD’s site, along with a diagnosis that pinpoints the specific gene needing repair in monogenic disorders, makes it possible for people around the world to connect and form patient advocacy groups to work for a cure. Remote participation in research and trials means that people with rare diseases can participate from wherever they are located, and Orphan Drug designation brings financial incentives for the creation of cures for small populations of patients. Today, we are already seeing movement toward a cure in some of the most well-known rare diseases as all of these factors combine with advances in gene and cell technology. The improvement of vectors and the use of CRISPR will likely lead to more.
Take Away
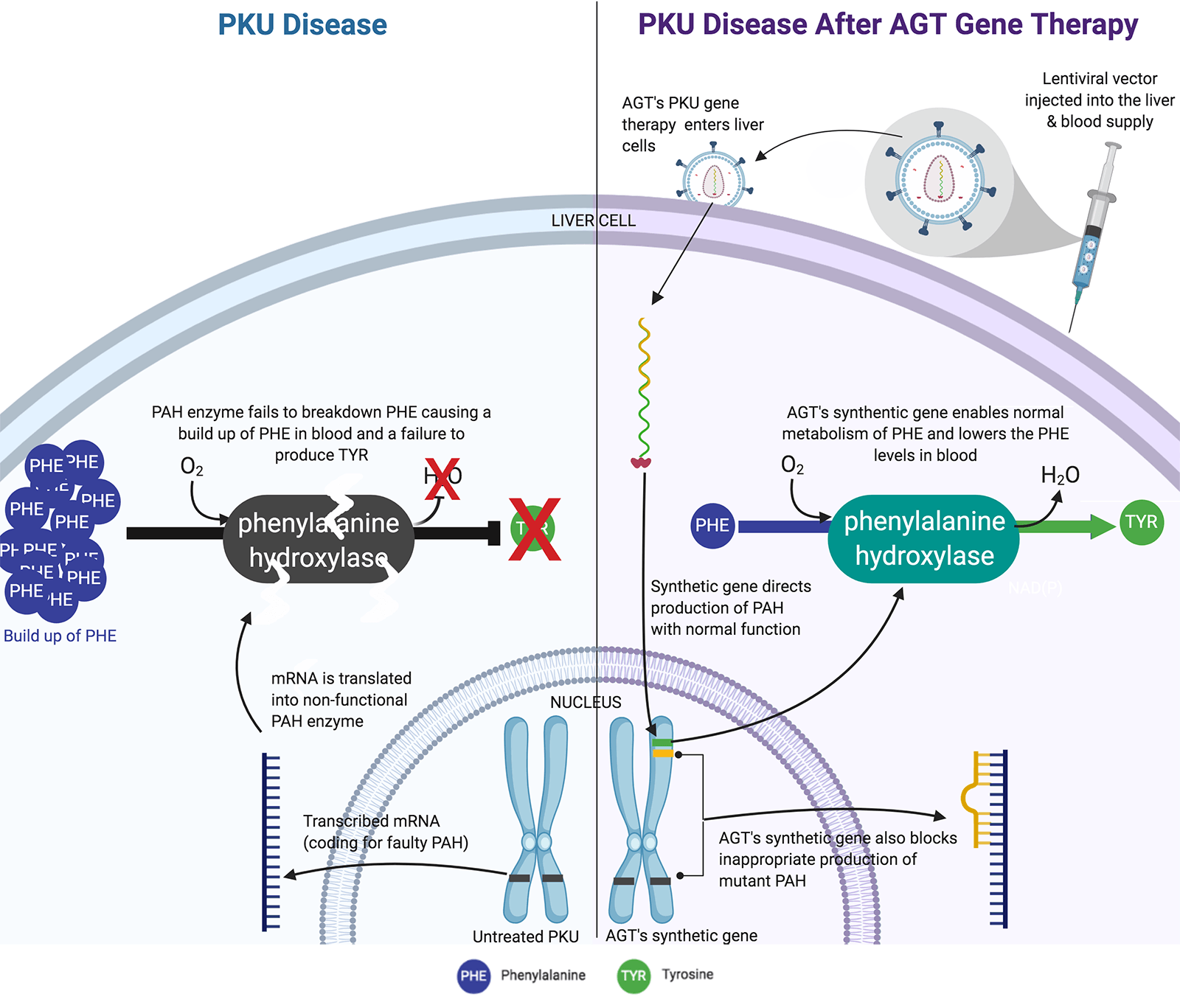
American Gene Technologies is committed to developing genetic therapies that will enable those with PKU to lead normal lives. The FDA granted AGT an Orphan Drug Designation in 2018. This designation provides AGT with eligibility for tax credits, market exclusivity for 7 years post-approval, and the waiver of new drug application fees totaling more than $2 million in cost savings. Today, AGT PKU Cure Program is in the pre-clinical validation stage.