9 Reasons Treatment of Rare Diseases Will be Big in 2025

Of the 7,000 known rare diseases affecting 350 to 400 million people worldwide, most have a genetic basis and it often comes down to a single gene that is wreaking havoc and harming the person’s health. Until recently, it was impossible to replace that one gene and save the patient. Much work has been done to map the human genome and develop techniques and tools necessary for gene therapy to realize the promise of treatment for these rare diseases. Here are nine reasons that the treatment of rare diseases will be big in 2025:
1. Remote participation will eliminate the need for travel for treatment
Rare diseases impact more people than cancer and AIDS combined. While we can say that about 1% of a nation’s population has a rare disease, altogether this is an immense number. Moreover, the American Journal of Managed Care (AJMC) reported that a 2019 study published in the European Journal of Human Genetics found that “approximately 4% of the total world population is affected by a rare disease at any given time, according to new research on 3,585 rare diseases.” The number of individuals with rare diseases can be quite large in many nations, with the emotional toll and cost of care reaching beyond the patient. New technologies in development will make it possible to reach rare disease patients remotely, without the need to physically travel to a different country for a trial. Harsha Rajasimha, Ph.D., founder and CEO of Jeeva Informatics Solutions Inc. described this in a previous article, Treatment of Rare Diseases: What You Need to Know. Rajasimha stressed the importance of remote participation since, without remote access, a participation depends on “the patient’s ability to travel and their zip code.”
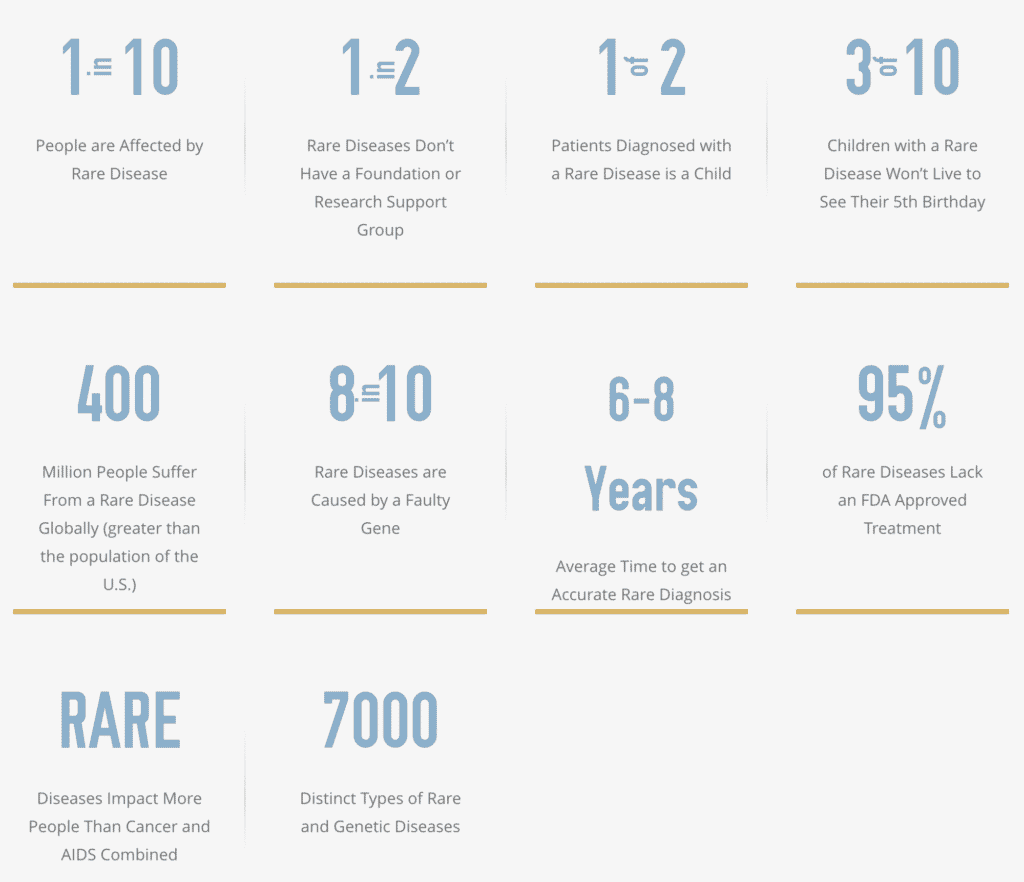
Credit: Global Genes - Allies in Rare Disease
2. It is now possible to determine the precise gene responsible for a monogenic disease.
One of the enduring benefits of the global effort to map the human genome is the ability to use the knowledge attained for a variety of purposes. Another important factor related to rare disease treatment is that there is a genetic basis for most rare diseases. Most are monogenic - diseases caused by an error in a single gene occurring in all cells of the body. Once identified, this error can potentially be cured with gene therapy. With the necessary information from gene sequencing in hand, the next step in creating a cure is to find a doctor or researcher with a thorough understanding of that specific genetic defect. Once that is accomplished, as was the case for Amber Freed, as we discussed in a previous article, the researcher will not only be familiar with the genetic condition but possibly have recommendations on how to treat it.
3. Orphan Drug Status provides many benefits
The Orphan Drug Act of 1983 made it possible for those with Orphan Drug Status to benefit from a range of incentives for those developing treatments for populations that meet the criteria. Irene Tennant, AGT’s VP of Clinical Product Development and Regulatory Affairs, discusses the tax credits, market exclusivity, and waiver of significant fees in-depth in Benefits of FDA Orphan Drug Designation: What You Need to Know.

Credit: American Gene Technologies
Researchers often know precisely what they must do to fix the error; they must replace the error with a working gene. “But for most point mutations that cause genetic diseases, simply cutting the already-mutated gene won’t benefit patients because the function of the mutated gene needs to be restored, not further disrupted,” said chemical biologist David Liu in his recent TED Talk. To solve this problem, Liu’s team developed molecular machines known as base editors. This new genome editing approach uses components from CRISPR systems to “directly convert one base to another without disrupting the rest of the gene.”.
The use of existing viral vector platforms and other technologies can speed the process of developing a treatment because researchers don’t have to start from scratch. It also reduces the costs associated with finding a treatment by using existing methods. AGT CEO Jeff Galvin says, “What we’ve done is create a platform of combinable components that can be mixed and matched to cure a myriad of diseases. It’s very much like maintaining a library of software subroutines. Such a collection of components makes it easy for you to create a solution on the existing foundation that already has the common elements of gene and cell therapy that span broad areas of disease control, starting the drug developer up to 80% of the way done on day one, and leaving them only the details that are particular to their target disease. It is sort of like an ‘iPhone for disease cures’ where curing a disease is like writing an app”.
6. “One-and-done” treatments make it possible to cure a patient.
One-and-done treatments are those treatments that take a single application to bring about a lasting effect or cure. By using viruses that carry DNA, gene therapy allows us, says Galvin, “to crack open these viruses, scoop out that viral DNA, and then replace it with other DNA that actually improves your health, improves the operation of the cell. We’re essentially converting viruses into updates for the operating system of your “self.” The result is a one-time treatment that replaces the incorrect gene forever.” The potential for such a cure will continue to put the focus on rare disease treatment.
One of the biggest problems with curing monogenic diseases is the immune response mounted by the patient’s own body. Known as graft vs. host disease, it can result in the death of the patient. This can occur when “you put a missing protein back in the body,” says Galvin, “because you’ve grown up all your life without that protein, your immune system will recognize that as an invader and try to attack it.” That is holding up a lot of work right now. Galvin says that researchers are “going to find a way to fix that, and that’s going to open the door to many different diseases.”
8. The other 20%
The good news is that gene therapy holds promise for other rare diseases. In Gene Therapy: More than Monogenic Disease, contributing author and Senior Biotechnology Analyst John Vandermosten provides a brief review of three programs using gene therapy for diseases that are not among the 80% that are monogenic. Vandermosten writes, “Gene therapy is a natural fit for addressing monogenic diseases; however, its application is much broader, and there are numerous programs underway that address other classes of disease in innovative ways.”
9. Gene therapy is no longer the stuff of science fiction or wishful thinking.
Treatment of rare diseases is within our grasp. As more work is done and more tools are created, the cost for each treatment will fall in the same way that the cost of desktop computers fell with ongoing innovation. For instance, Galvin recounts in his webinar, Cell and Gene Therapy: Discovering the Clinical & Commercial Potential, that in the same way smartphones have become available the world over as prices decline, gene therapy will become as ubiquitously available as the technology matures and moves down the cost curve.
Why 2025?
There are many factors in play that will make 2025 a big year for the treatment of rare diseases. Access to trials will become available to more patients through remote participation. Identification of the precise gene responsible for monogenic diseases will make it possible to explore life-saving treatments. The benefits obtained through the Orphan Drug Designation for pharmaceutical companies developing treatments will continue to be a positive factor. The combined use of base editors that can correct a point mutation, along with platforms and tools that are readily available can bring companies almost all of the way towards a successful treatment on day one. Combine this with one-and-done treatments that can be lifesaving, and in a few years it will be feasible to tackle these rare diseases in an efficient, cost-effective, and productive way. All told, today’s science and technology achievements hold great promise for the treatment of rare diseases in 2025.
AGT sponsored this article to provide information about research in rare disease treatment. Learn more about AGT’s work in rare disease treatment through AGT’s Phenylketonuria Cure Program and Orphan Drug Designation.
Choosing Lentivirus Vectors for PKU Therapy
Modern gene therapy relies on viral vectors for delivering genetic medicines to sites of disease. The field of viral vectors includes a surprising range of alternate approaches, but current clinical applications mainly use lentivirus (LV) or adeno-associated virus (AAV) vectors. These two platforms are distinct in many ways and vector choice will impact all stages of clinical investigation, product licensure and uptake by the PKU patient population.






