ARC of HIV History 2 – A Beginning and an End


Contributing Author John Vandermosten, Senior Biotechnology Analyst
This is the second installment of our series on the Arc of HIV History which examines the earliest emergence of HIV and the progress that has been made until today. In Part 2 we look at the genesis of the first approved HIV drug and the first cure of HIV in the Berlin Patient.
The First Salvo
In the wake of the John F. Kennedy assassination and two years before the Berlin Patient was born, the volatile year of 1964 saw US involvement in Vietnam increase and tremendous strides made in civil rights. It also produced the first drug that would be later used to treat HIV. American scientist Jerome Horwitz, while working at the Karmanos Institute in Michigan, created a compound intended to treat cancer called azidothymidine (AZT). He and others at the time had theorized that retroviruses like HIV caused most cancers. With a grant provided by the National Institutes of Health, Horowitz designed the drug to integrate counterfeit nucleotides into cancer cell DNA to stop their growth. The drug interferes with the enzyme reverse transcriptase, preventing a virus from replicating. Despite extensive animal model work, AZT did not show any activity against cancer in early testing and the compound was set aside as Dr. Horowitz pursued greener pastures.
President Johnson Signing the Civil Rights Act of 19641

Retroviruses infrequently affect humans and, due to their rarity, had not received much attention from researchers in the decades after Horowitz’ discovery. A retrovirus is a type of virus that uses RNA as its genetic material and relies on reverse transcriptase to replicate within a cell. This changed after Drs. Barré-Sinoussi and Montagnier (which we discuss in our first installment) at the Pasteur Institute discovered that HIV was indeed a retrovirus. Now that scientists understood the mechanism underlying the infection, efforts were made to find a treatment. Scientists sought to block the activity of reverse transcriptase and a search was made far and wide to find an answer.
In 1984, Burroughs Wellcome virologist Marty St. Clair was involved in a project that examined the effect of blocking reverse transcriptase in HIV. The effort screened infected cells in the presence of numerous compounds searching for a candidate that was able to halt HIV replication in the lab. She recounts her work placing living cells in petri dishes and adding virus. Her role was to examine each petri dish to identify a clear area in a field of virus, or plaque, which indicated activity against the virus. Upon coming to the plates that had been treated with AZT she recalled that “I was holding them up to the window, I came to 16 dishes, none of which had any plaques."2 This exciting moment was the beginning of an accelerated three-year effort to gain approval for the drug.
Dr. Marty St. Clair (R)3

Clinical Trials and an Approval
Building off of St. Clair’s work, Burroughs Wellcome launched a study of the nucleoside analog reverse transcriptase inhibitor that included 282 HIV-positive patients in a placebo-controlled, randomized, double blind study. The trial was stopped early due to substantially higher efficacy observed in the active group where only one AZT patient died in contrast to 19 patient deaths in the placebo arm. Despite not having generated long-term safety data, the drug was fast tracked through the approval process and given the green light by the FDA in March 1987. In the months and years following the approval, criticism was launched against the FDA and Burroughs Wellcome for many reasons including the cost of the drug, ownership rights, safety, rigor of the trial, short benefit duration and lack of follow up. In later work, the initially approved dose was reduced and AZT was later combined with other treatments into Combivir4 and Trizivir5 which provided beneficial synergies. Despite all of these difficulties, AZT became the first treatment available for HIV and gave scientists, physicians and patients hope that more would be coming.
Building on the Foundation
Another milestone in the drug development effort against HIV was the introduction of Highly Active Antiretroviral Therapy or HAART. This approach combines multiple antiretroviral drugs employing different mechanisms to halt HIV from reproducing, thereby maintaining a low viral load. HAART commonly includes three or more drugs to treat HIV infection. AZT was originally part of this drug cocktail but was replaced in most cases with safer and more effective options. Some of the classes of antiretroviral drugs in HAART include:
- Nucleoside reverse transcriptase inhibitors (NRTIs)
- Non-nucleoside reverse transcription inhibitors (NNRTIs)
- Protease inhibitors (PIs)
- Entry or fusion inhibitors
- Integrase inhibitors (INSTIs)
This approach is effective because if the virus builds resistance to one of the agents, the others can continue to inhibit it. HAART treatment has been successful compared to previous approaches and has reduced viral load to undetectable levels and has essentially arrested the progress of the disease. HAART was an impressive achievement which allowed HIV patients to live a nearly normal life span. However, side effects are severe and include bleeding, bone loss, heart diseases and numerous other conditions. The regimen must also be taken according to a strict schedule and can cost from $25,000 to $50,000 per year.6 While a material improvement over what had come before, this was still not a cure.
At Last, What We’ve Been Waiting For
In 2007 an event that would later shock to the infectious disease community took place: a functional cure for HIV was found. It was not easy, cheap or even appropriate for all but a small minority of HIV patients, but it was the first time HIV had been permanently suppressed below detectable levels in a patient.
The patient at the center of this electrifying event was originally from Seattle, Washington. Timothy Ray Brown grew up in a single-parent household and at an early age took part in activism on behalf of people with AIDS in high school. After graduation, he expanded his horizons and moved to Barcelona, then later travelled to Germany to pursue academic studies. Life was exciting; however, HIV was spreading throughout the world, and 1990s Berlin was not immune from the risks of exposure to the virus. After a call from a former lover who disclosed that he was HIV positive, Timothy went to be tested and was later diagnosed with HIV in 1995. Standard treatment at that time was AZT but HAART became available shortly after and he underwent anti-retroviral therapy over the next decade.
Timothy’s HIV had been successfully suppressed, but, in 2006 during a trip to New York, his health began to worsen and after returning to Berlin he was diagnosed with acute myeloid leukemia (AML). First line treatment for AML is chemotherapy; however, after a short period of remission, the treatment was unsuccessful and other options were considered. Mr. Brown’s oncologist, Dr. Gero Hütter, wanted to pursue a novel approach and perform a stem cell transplant using a donor with the CCR5 Δ-32 deletion, the same mutation that prevents HIV from entering immune cells. He speculated that it might be possible to replace his patient’s immune system with a new one that could not be infected with HIV. Dr. Hütter searched the German stem cell database for donors with the desired characteristics and prepared his patient for the difficult procedure.
A stem cell transplant is a risky approach given an average one-year survival rate below 70%.7 It is considered for AML patients when chemotherapy fails, and other treatments are not appropriate. In an allogenic transplant like the one that Timothy Ray Brown received, the procedure requires a human leukocyte antigen (HLA) match between the donor and the recipient. There are two ways that stem cells can be contributed, either using peripheral blood stem cells (PBSCs) or bone marrow. A donor provides the stem cells which are harvested from PBSCs or from bone marrow through a small needle puncture in the pelvic bone. Then the cancer patient must have their existing immune system purged with whole body irradiation and/or high dose chemotherapy through a process called conditioning to prepare for the transplant. Conditioning reduces the risk of rejection, eliminates existing disease and removes the defective blood stem cells. It also leaves the patient without an immune system. Donor stem cells are then infused, and the stem cells engraft and begin to multiply and make new blood cells in a process that can take about a month, rebuilding the body’s ability to fight infection. Serious complications can arise including graft versus host disease, infection, pneumonia among many others.
Stem Cell Transplant 8
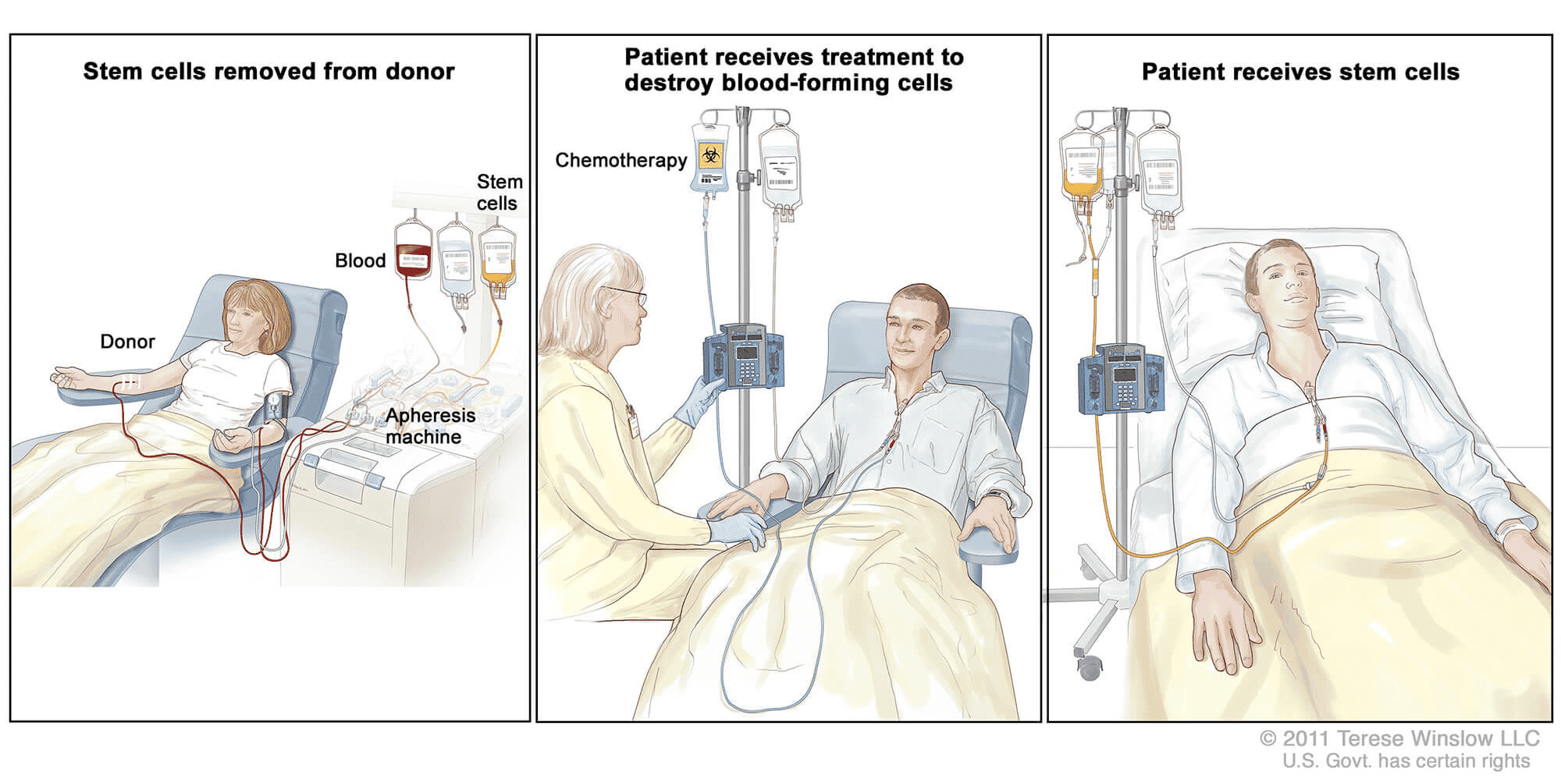
In Timothy Brown’s case, an appropriate donor with the CCR5 Δ-32 deletion was found. A team of doctors performed the transplant and after three months of recovery, the AML appeared cured and HIV was no longer detectable. However, less than a year later, in early 2008, Mr. Brown suffered a recurrence of the cancer and another stem cell transplant was performed. Recovery from the second transplant was very difficult, as he was temporarily paralyzed, nearly went blind, suffered from delirium and required a brain biopsy. After an extended recovery that included rehabilitation for brain injuries, the cancer went into remission and Mr. Brown continued to show no sign of HIV in his system.
The Berlin Patient’s Legacy
A paper was published in the New England Journal of Medicine in 2009 detailing the stem cell transplant procedure and successful suppression of HIV penned by lead author and Mr. Brown’s oncologist, Gero Hütter. The subject of the paper was initially known as the Berlin patient, but in 2010, Timothy decided to go public and release his name and picture to the media. He felt that his story could be an inspiration to other patients with HIV and to scientists and doctors that continue the effort for a cure. Mr. Brown started the Timothy Ray Brown Foundation under the World AIDS Institute and spoke on countless occasions at conferences and HIV/AIDS events promising to “not stop until HIV is cured!”9
After 11 years of remission Timothy Ray Brown died on September 29, 2020 of leukemia in Palm Springs at age 54. Mr. Brown spent his last decade sharing his success story with other HIV patients and scientists to inspire an improved cure for the disease. He leaves behind a legacy of perseverance and service that continue to motivate others to take up the fight against this terrible disease.
Dr. Gero Hütter (L) and Timothy Ray Brown (R)10

Please join us again soon for our next installment of the Arc of HIV where we will discuss some of the early attempts using gene therapy to address HIV which built on the successes and learnings from the stem cell cure.
1 Source: Lyndon B. Johnson Library and Museum; photograph, Cecil Stoughton
2 http://news.bbc.co.uk/2/hi/health/7117651.stm
3 Viiv Healthcare webpage accessed October 2020. https://viivhealthcare.com/en-gb/our-stories/our-people/treating-hiv-building-on-30-years-of-innovation-ingenuity-and-passion/
4 Combivir is a combination of 150 mg of Epivir (3TC) and 100 mg of Retrovir (AZT) approved in 2000.
5 Trizivir is a combination of three previously approved drugs: 300mg of Retrovir (AZT), 150mg of Epivir (3TC), and 300mg of Ziagen (abacavir) approved in 2002.
6 Shaw, Maggie. What is the true cost of the high price of ART? The American Journal of Managed Care. https://www.ajmc.com/view/what-is-the-true-cost-of-the-high-price-of-art
7 Reported survival rates are extremely broad ranging from 30% to almost 90% over varied time periods. Rates have improved in recent years and are also impacted by whether the transplant is autologous, allogenic, and the degree of closeness of the allogenic donor among other factors. Patient age also plays an important role. We note that Dr. Hütter tried the stem cell transplant approach on six other HIV cancer patients and none of them survived longer than one year as reported in a letter to the editor of the NEJM.
8 Source: National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/types/stem-cell-transplant
9 Brown, T.R. I Am the Berlin Patient: A Personal Reflection. AIDS Res Hum Retroviruses. 2015 Jan 1; 31(1): 2–3.
10 Fred Hutch Website, Doctor who cured ’Berlin Patient” of HIV: ”We knew we were doing something very special.” February 27, 2015. https://www.fredhutch.org/en/news/center-news/2015/02/timothy-ray-brown-doctor-who-cured-him.html





