5 Biotech Companies in Maryland That May Make History in 2021

Contributing Author: Gina Hagler
These five biotechnology companies in Maryland may make history in 2021 because they have multiple therapeutic programs in trials for a variety of genetic diseases.
#1

REGENXBIO is a publicly-traded company that develops products for one-and-done, single-dose solutions addressing genetic defects. Their products use adeno associated virus (AAV) viral vectors that make up their proprietary NAV Technology Platform, which utilizes novel AAV (NAV) for retinal, metabolic, and neurodegenerative diseases. To accomplish this, the NAV Vectors deliver genes to cells, making it possible for cells to produce the proteins that will impact the disease.
Platform
The NAV Technology Platform is a patented set of AAV (adeno-associated virus vectors), which neither replicate nor are known to cause disease. The REGENXBIO NAV Vectors deliver a healthy copy of the gene, located inside the AAV capsid. As described in this video, once inside the body, the capsid travels to the targeted tissue where the NAV Vector inserts the healthy copy of the gene into a cell before dissolving. The inserted DNA is transcribed into RNA in the nucleus of the cell, which encodes the missing protein. As this gene continues to replicate, it produces sufficient quantities of the essential protein to impact the disease.
Pipeline
The REGENXBIO pipeline includes product trials ranging from preclinical to Phase II for four program areas. The company has worldwide rights to ten of these, with co-commercialization for one. Of particular interest are the Fast Track Designations granted for neurodegenerative diseases and the Orphan Drug Designation for one retinal disease and three neurodegenerative diseases because these bring an advantage to those trials. The Rare Pediatric Disease Designation for four of the trials is equally significant because of the financial benefits that go with the designation as well as the fact that these treatments will fill an unmet need for a portion of the 350 million people worldwide with one of 7,000 rare diseases. In addition, the REGENXBIO trials are for a mix of AAV-mediated antibodies for chronic diseases (6) and monogenic gene replacement, providing more than one area of opportunity for REGENXBIO.
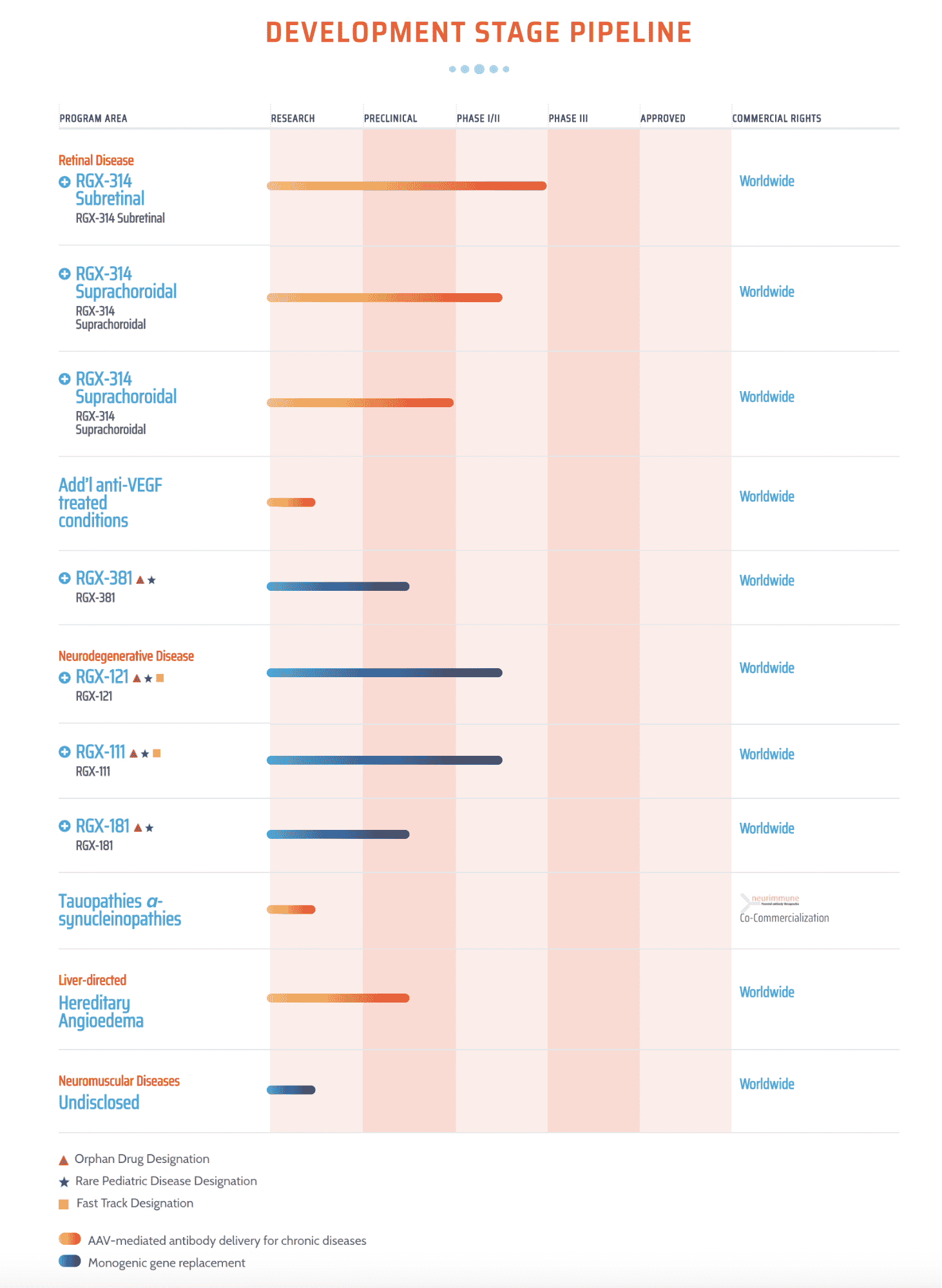
REGENEX Development Stage Pipeline
Source: REGENXBIO, 2020
Promise
REGENXBIO (NASDAQ: RGNX), located in Rockville, uses its proprietary NAV Vector Platform with a range of treatments in trials that are at various stages. The products in development meet a variety of patient needs, while the company retains worldwide rights for these products that will serve a global population that has significant unmet needs.
#2

Precigen is a publicly-traded biopharmaceutical company working in the discovery and clinical-stage space with both cell and gene therapy programs. Their focus is on the urgent and intractable diseases in immuno-oncology, autoimmune disorders, and infectious diseases. The company is pursuing multifunctional gene and cell therapy that will work together for more efficacious, safer treatments.
Platform
Precigen has a proprietary viral vector, the AdenoVerse with several advantages when compared to other vectors. These include a/an:
- Capacity to carry a larger genetic payload - significant because it makes it possible to include more than one gene for expression, as well as because it makes it possible to deliver the large gene required for some genetic disorders without having to manipulate that gene to accommodate the smaller capacity of the viral vectors currently available.
- Ability to repeat the treatment - significant because with many adenoviral vectors, there is a concert that the patient builds antibodies that make a second treatment with that vector impossible.
- Lack of replication - significant because the fact that there is no in vivo replication reduces the risks associated with the therapy.
- Durable response - significant because the antigen-specific immune response is lasting.
Their UltraVector platform blends gene therapy technologies with computational models to incorporate genetic components into an optimized expression of multiple genes. Their methodology and library of characterized genetic components and associated functional characterization data are the foundation of this platform.
Precigen also has a product known as the ActoBiotics Advantage. This is a food-grade bacteria that makes it possible to deliver biologics via mouthwash, capsule, or a topical formulation. The bacteria, L. lactis, has a long history of safe use. The process for inserting and delivering the therapeutic agent is described in the illustration below.
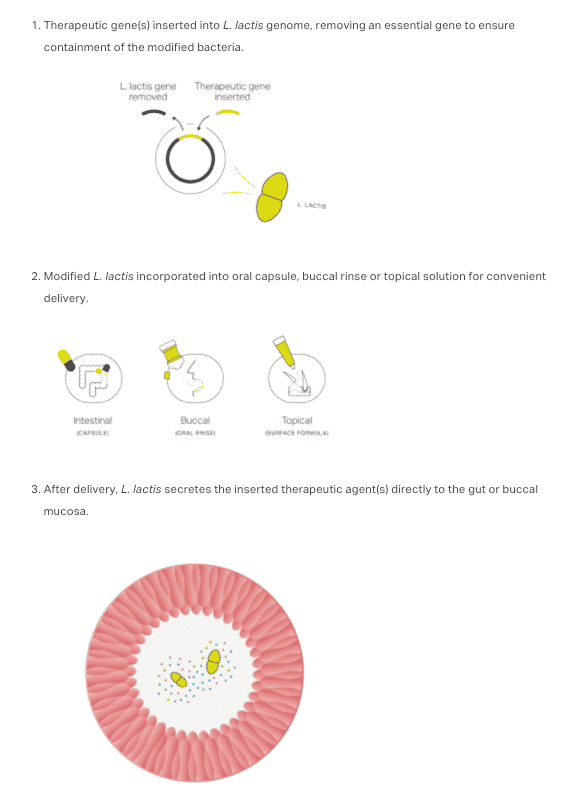
Source: Precigen, 2020
Pipeline
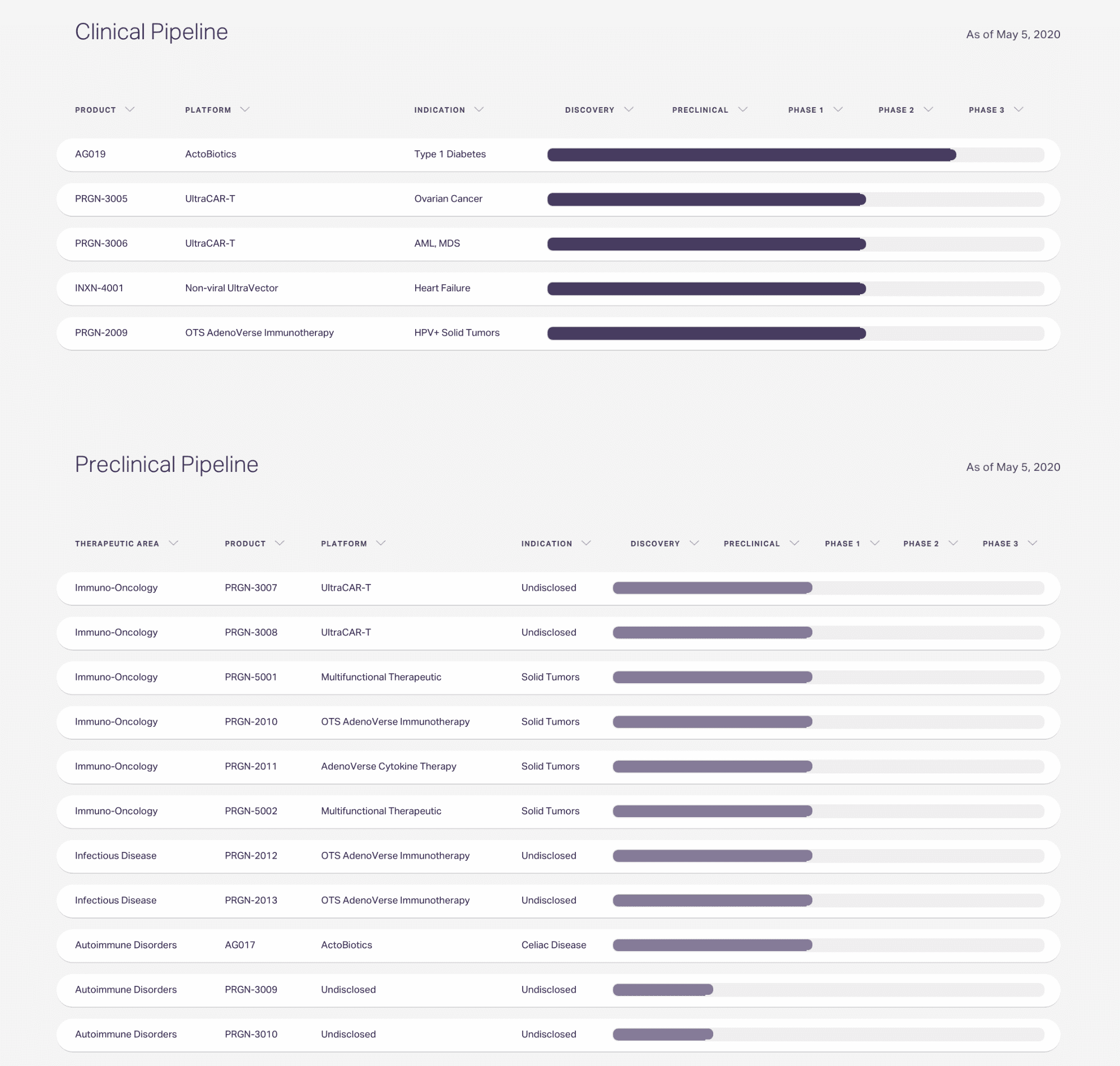
Precigen Development Stage Pipeline
Source: Precigen, 2020
Precigen’s pipeline includes therapies in both clinical and preclinical stages. Their gene therapies in clinical trials include one for Type 1 Diabetes using ActoBiotics as a delivery mechanism and one using non-viral UltraVector biotechnology for heart failure. In the preclinical stage, the company is exploring therapeutic products for solid tumors, infectious diseases, and celiac disease.
Promise
Precigen (NASDAQ: PGEN), located in Germantown, has a range of treatments in its pipeline. Several are currently in clinical trials, with many more in the preclinical phase. It is encouraging that Precigen has many promising therapies based on their products and platforms. Their larger vector, and their alternate delivery route for biologics, differentiate the company’s therapies and puts Precigen in the position to make history in 2021.
#3

MacroGenics is a publicly-traded biopharmaceutical company that develops and commercializes monoclonal antibody-based therapeutics for cancer. In December 2020, MacroGenics received FDA approval for Margenza, a targeted therapy to be used in conjunction with chemotherapy to treat patients with metastatic HER2-positive breast cancer. It is the first FDA approval for MacroGenics.
Platform
The company has two platforms, DART and TRIDENT, that make it possible to create single-molecule medicines that simultaneously bind to two or more targets with antibody-like specificity. The result is an environment that produces a more significant biological effect than other forms of targeted binding.
Pipeline
MacroGenics has 7 products in their pipeline. Two are in the pre-approval stage and the others are in various stages leading to possible approval. The company has major market rights as indicated in the table below.
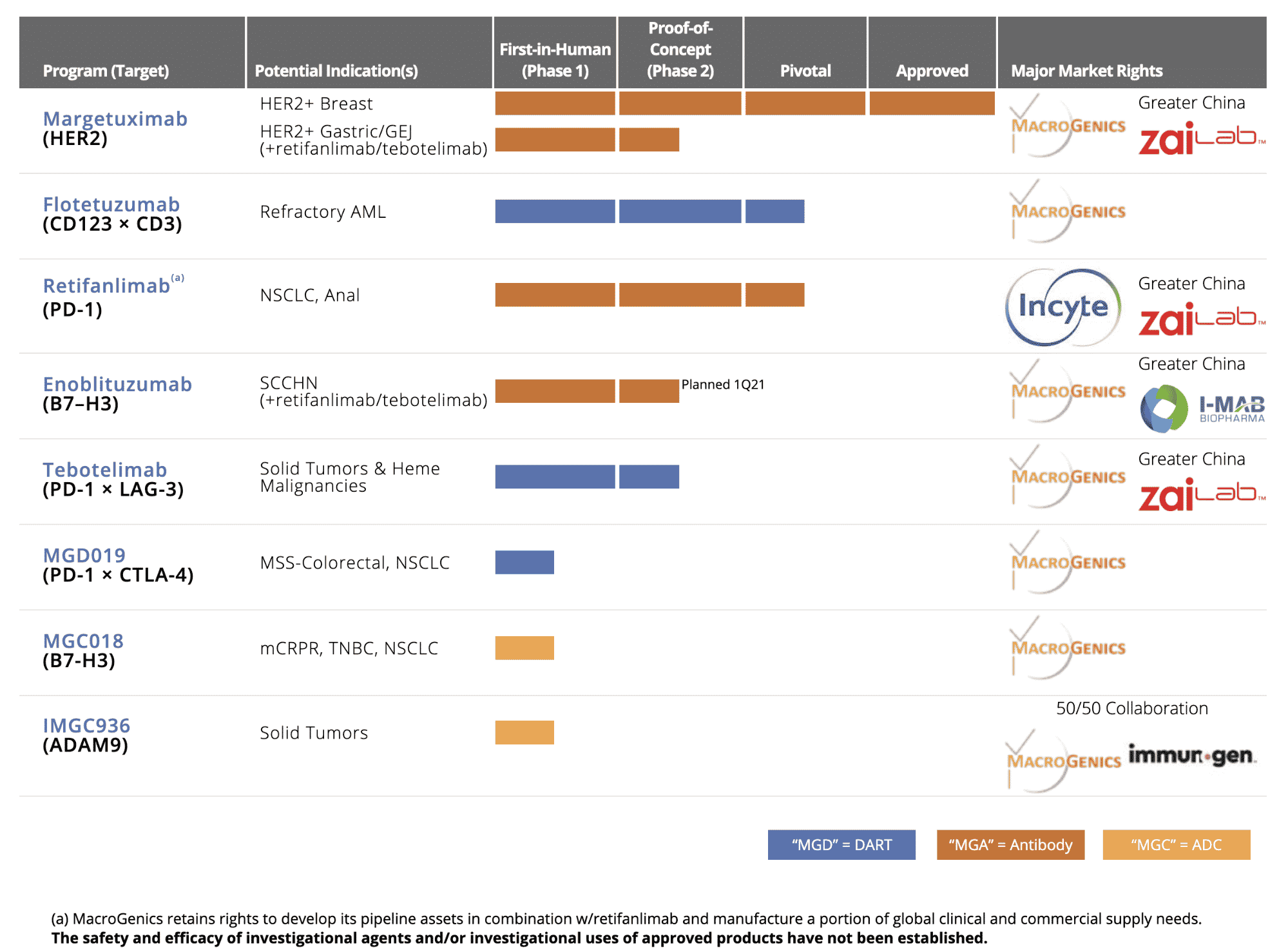
MacroGenics Development Stage Pipeline
Source: MacroGenics, 2020
MacroGenics also holds more than 30 patents for product candidates relating to hematological malignancies and solid tumors.
Promise
MacroGenics (NASDAQ: MGNX), located in Rockville, may make history in 2021 due to FDA approval of Margenza. Flotetuzumab for refractory AML and Retfanlimab for NSCLC, Anal are nearing approval. These therapies, in addition to the others in Phase 1 of clinical trials and the many patents the company owns, positions MacroGenics for history-making approvals and commercialization in 2021.
#4

OpGen is a publicly-traded biotechnology company in the gene therapy sector. Their focus is on genomic diagnostics to reduce the spread of infections caused by multidrug resistant microorganisms (MDROs).
Platform
OpGen has a suite of products designed to ensure OpGen a position as what the company calls “a steward for today’s antibiotics.” Their platform includes tools to predict, track, and identify drug-resistant microbes. Two of them are cloud-based and offer identification of pathogens in a few hours rather than several days:
- Acuitas Lighthouse is the first cloud-based software working at the molecular level with genotype and phenotype data collected from hospitals around the world. By working at the molecular level, OpGen is able to identify, track, and predict antibiotic-resistant infections. The information generated by Acuitas Lighthouse can be used by healthcare systems for real-time treatment and mitigation decisions.
- Acuitas Tests is their proprietary testing panel. It can return pathogen identification in hours, rather than days by using Merck & Co’s Study for Monitoring Antimicrobial Resistance Trends (SMART) surveillance program - a curated library of more than 250,000 bacterial isolates.
Pipeline
OpGen does not have a pipeline of therapies because its business model uses proprietary platforms to save lives around the globe. This is accomplished by reducing the number of deaths each year from undiagnosed bacterial infections or drug-resistant infections. Their approach is not only diagnostic but also uses AI to suggest treatments to healthcare workers in real-time. OpGen is working to prevent the number of people affected as indicated below.

OpGen’s Target Markets
Source: OpGen, 2020
Promise
OpGen (NASDAQ CM: OPGN), located in Gaithersburg, has developed a cloud-based system that integrates genomic information from healthcare partners with rapid testing of patients for pathogen identification and AI suggestions for treatment in real-time. The market for its service is global. With increasing alarm at the number of antibiotic-resistant infections, OpGen is poised to reap the benefits of its technology and make history in 2021.
#5

American Gene Technologies (AGT) is a private biotechnology company developing genetic medicines through the application of proprietary viral vector technology. Its programs are focused on an HIV functional cure, effective gene therapy treatment of phenylketonuria (PKU), and immuno-oncology products to bring about cancer cures.
Platform
AGT’s strength lies in its viral vectors. These vectors have been developed, tested, and banked for use with gene therapy for specific diseases. When a company approaches AGT with an approach, AGT pairs the requirements for that approach with the appropriate vector. This leaves the researchers as CEO Jeff Galvin says, “80% of the way on day one.” By focusing on the delivery of the genetic cargo, AGT has created a layer in the tech stack that will be needed by all firms intending to deliver gene therapy.
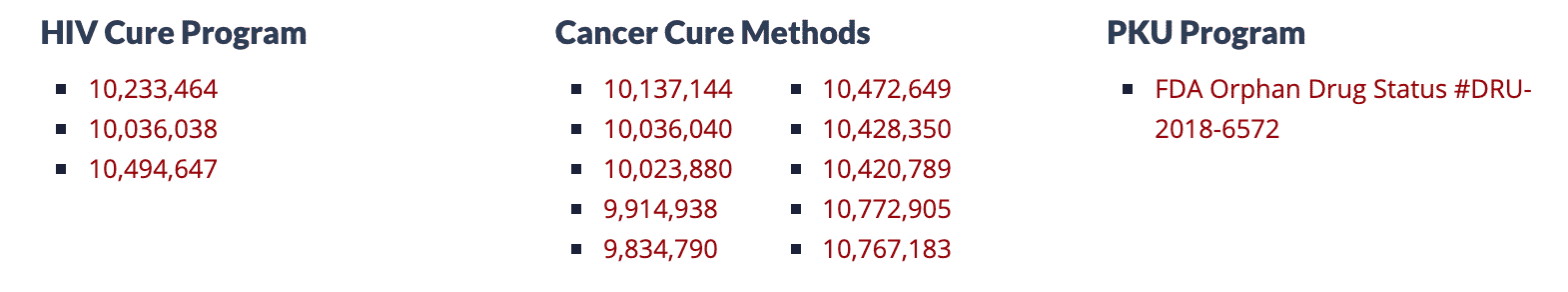
The company also holds several patents related to its programs.
Pipeline
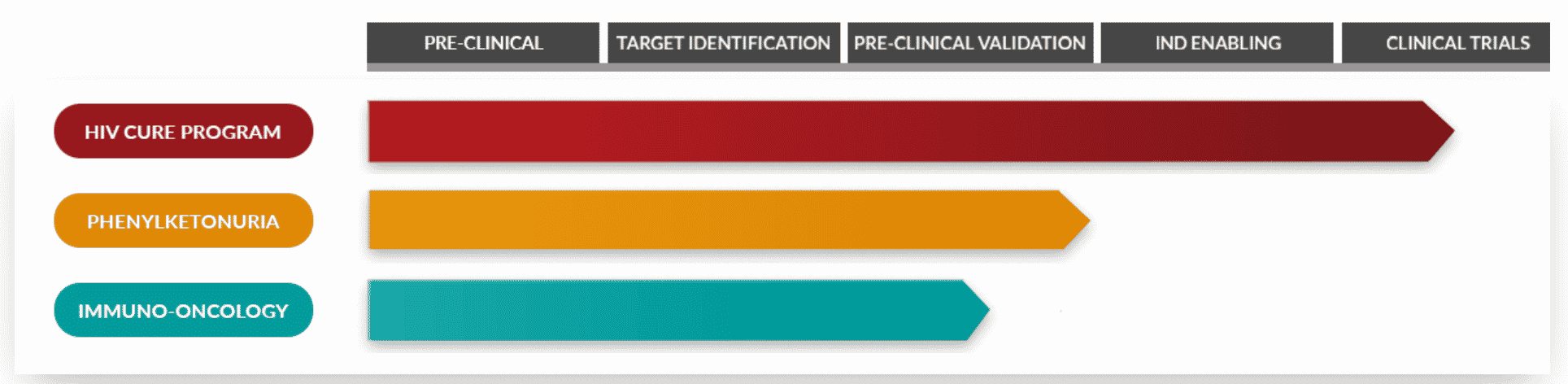
AGT Development Stage Pipeline
Source: AGT
Currently, AGT is beginning the first phase of clinical trials for its HIV Cure Program. Early indications suggest that this cell therapy will be the first successful one-and-done therapy resulting in a functional cure for those with HIV. For the millions of individuals living with HIV, this will mean the end of ongoing treatment. The PKU program is a gene therapy that will introduce a functioning gene to replace the mutated gene at the heart of PKU. Again, this is intended to provide a one-and-done cure. The immuno-oncology program is developing a therapy that will activate innate T cell immunity to kill all tumor cells with its vector.
Promise
American Gene Technology, located in Rockville, has over 10 patents and an Orphan Drug Designation for its programs. With Orphan Drug Status for the PKU Program that is currently nearing the IND stage and the HIV Cure Program in Clinical Trials. If its HIV Cure Program is successful, AGT will have the first functional cure for individuals living with HIV.