Top Cell Therapy Approvals and Pipeline / Cell Therapy is More Than CAR T

Contributing Author John Vandermosten, Senior Biotechnology Analyst
Cell therapy is the transfer of therapeutic cells to a patient. It replaces damaged cells or enhances normal cells with modified new ones.[1] The cells used in the process may be autologous or allogeneic; however, they must be recognized by the immune system in a way that will promote the desired response. Some cell therapies modify the target so that it can provide an enhanced or tolerogenic immune function for cancer, infectious disease or autoimmune disease. Other cell therapies use pluripotent, multipotent or differentiated cells in regenerative medicine, depending on the type of treatment. The space has a broad definition that matches its extensive potential.
Cell therapies have been around a long time. Some of the earliest recorded examples of using tissue to improve health were from Roman times when physicians performed exsanguination and reinjection. We have come a long way since then with the first successful blood transfusions taking place in the early 19th century[2] followed a century and a half later by the first successful bone marrow transplants. Advances in cell therapy accelerated during this period with physiologist Charles Édouard Brown-Séquard conducting early work in transfusion, even injecting himself with animal cells to counter the effects of aging in the 1880s.[3]
After decades of substantial growth in medical learning, stem cells rose in prominence in the scientific community in the 1960s with seminal work done by Ernest McCulloch and James Till. Their research was in part inspired by the hematopoietic stem cell transplantation first performed by Georges Mathé in 1958. This early work used embryonic stem cells, which raised ethical issues due to the destruction of the embryo. Evolving beyond embryonic cells, Nobel Prize winner Shinya Yamanaka and John Gurdon advanced the field substantially with their work on stem cell differentiation showing the potential for mature cells to become pluripotent. Bridging to the present period, Carl June is yet another luminary in the field, who laid the ground work for chimeric antigen T cell (CAR T) therapy. His work provided the foundation for the first approval in this class of medicine and he discovered a way to fight against cytokine release syndrome which is one of the major drawbacks of CAR T. CAR T has generated impressive efficacy for blood cancer patients and sets the stage for more therapies that can use modified cells to improve health.
Cell therapy is sometimes overshadowed by more popularized approaches such as CRISPR[4] or gene therapy, but there is one type of cell therapy that has been well publicized. This is chimeric antigen receptor T cell or CAR T therapy. Bursting on the scene in August 2017, Novartis’ Kymriah became the first approved CAR T therapy followed two months later by approval of Kite Pharma’s Yescarta. These breakthrough therapies improved success rates in certain types of lymphomas providing full remission in up to 90% of patients. But there is more to cell therapy than CAR T. It can be as simple as blood transfusion, but can also include regenerative medicine, genetic modification, stem cell therapy, tumor infiltrating lymphocyte (TIL) therapy, and many others some of which we share in this article.
Public Company Innovators in Cell Therapy
Work performed by the scientists and leaders in cell therapy have spawned further advances which have been taken up by the many innovative companies in the field. The first cell therapies were blood transfusions and bone marrow transplants, but as time passed, stem cell therapies advanced to treat type 1 diabetes, Parkinson’s disease and spinal cord injuries. In some cases, cells are modified using gene therapy to either correct a mutation or add a feature. Below we share some of the leading technologies in cell therapy and the companies that are moving them forward.
Capricor Therapeutics (NASDAQ: CAPR)
Capricor is a cell and exosome-based platform company that is developing two products. Its cell therapy is derived from donated heart muscle cells and designated CAP-1002. The allogeneic cardiosphere-derived cells do not engraft into host tissue, but rather excrete exosomes. The exosomes contain micro RNA (miRNA), non-coding RNAs and proteins that are internalized by target cells. They stimulate changes in the cell, reducing oxidative stress, inflammation and fibrosis while increasing cellular energy and muscle cell generation.
CAP-1002 is in Phase II clinical trials for Duchenne Muscular Dystrophy (DMD). DMD is an inherited disorder of progressive muscle weakness that predominantly occurs in boys due to alterations in a protein called dystrophin. It is both a genetic and rare disease that is estimated to occur in every 16 to 20 male births of 100,000. Final results for the Phase II study were presented September 2021 at the World Muscle Society Virtual Congress (WMS).
Athersys (NASDAQ: ATHX)
Athersys is a cell therapy and regenerative medicine company advancing MultiStem. MultiStem is an off-the-shelf stem cell therapy platform that does not require tissue matching or immunosuppressive drugs prior to administration. Source material comes from the bone marrow of adult donors, specifically Multipotent Adult Progenitor Cells (MAPC). The cells may be expanded outside the body and be re-administered to promote healing and tissue repair along multiple axes. The technology works through multiple mechanisms that down-regulate severe inflammatory response and stimulate reparative mechanisms that promote healing and recovery.
Athersys’ most advanced program is in ischemic stroke and is designated MASTERS 2. MASTERS 2 is a pivotal Phase III study that is seeking to evaluate outcomes based on measures of disability and dependence in patients experiencing stroke. Athersys is conducting additional trials in trauma, hematopoietic stem cell transplant, acute respiratory distress syndrome and acute myocardial infarction among others all using its MultiStem stem cell product.
SQZ Biotech (NYSE: SQZ)
SQZ Biotech offers a novel approach to delivering antigens and other cargo to target cells using its Cell Squeeze technology. The process forces cells through a constrictive microfluidic chamber, which deforms the cell thereby opening pores on its surface. The open pores allow payload to enter through diffusion including proteins, peptides, nucleic acids, ribonucleoprotein complexes and large molecules. After entry, the inserted material can promote a targeted immune response or stimulate immune tolerance. The process works with multiple cell types including T cells, B cells, monocytes and natural killer cells.
Exhibit I – Cell Squeeze Technology[5]
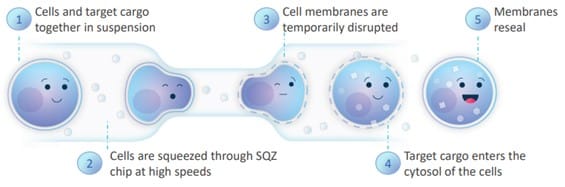
SQZ is researching and developing the use of its modified cells in human papilloma virus (HPV) indications inserting both antigen presenting cells (APCs) and activating antigen carriers (AACs) to activate an immune response. Two Phase I clinical trials are underway for HPV positive solid tumors using the APC and AAC platforms. Preclinical areas of interest include infectious disease and immune tolerance. The company has a partnership with Roche which has provided upfront payments and the promise of future milestones and royalties if the oncology programs are successful.
Adaptimmune Therapeutics plc (NASDAQ: ADAP)
Adaptimmune is a UK-based T cell receptor (TCR) company using its Specific Peptide Enhanced Affinity Receptor (SPEAR) platform to modify T cells so that they can better recognize tumor cells. These modified immune cells can recognize peptide fragments from proteins expressed both on the inside and outside of the cell. Manufacturing of the cells requires white blood cells from patients that is shipped to the company’s manufacturing facility. Magnetic micro beads are added to the cells to bind to CD3+ T cells and activate them in preparation for expansion in a bioreactor. The processed cells are then shipped back to the patient and infused into the patient for treatment.
Exhibit II – SPEAR Platform Manufacturing Process[6]
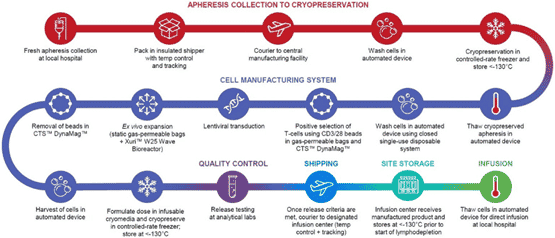
Adaptimmune is developing a broad platform of cell therapy candidates in various stages of clinical advancement. It offers four therapies including lead candidate afamitresgene autoleucel for synovial sarcoma and head & neck cancer in combination with pembrolizumab. The other programs are MAGE A4 in the SURPASS trial for esophageal cancers, AFP for hepatocellular carcinoma and MAGE A10 for the solid tumors with cancer testis antigens. The company’s next steps are to move its candidates to pivotal trials in preparation for regulatory submission.
Gamida Cell Ltd. (NASDAQ: GMDA)
Gamida Cell targets blood cancers and serious blood diseases using its Omidubicel therapy. Omidubicel is an advanced allogeneic cell therapy for transplanting bone marrow in hematologic malignancies. The product received a Breakthrough Therapy and Orphan Designation from the FDA. Phase III trials are complete and the company plans to submit a Biologics License Application (BLA) to the FDA in the second quarter of 2021. If approved this would be the first FDA approved cell therapy for bone marrow transplantation. In addition to Omidubicel, the company has five other candidates targeting blood cancers and solid tumors in its GDA series of products. These programs range from discovery stage, Phase I and Phase II level assets.
Gamida’s cell therapy approach uses nicotinamide to expand cells, such as stem cells and natural killer cells, while maintaining the original phenotype and potency of the cell. The resulting output has improved engraftment time and has resulted in a lower infection rate. Gamida’s approach is able to overcome the shortcomings of other approaches in this space such as a cells’ ability to traffic, localize and proliferate in vivo.
NexImmune, Inc. (NASDAQ: NEXI)
NexImmune uses its Artificial Immune Modulation platform to modulate the role T cells play to treat cancer, autoimmune diseases and infectious diseases. The company uses a precision approach to construct synthetic dendritic cells in the form of nanoparticles to direct a T cell mediated immune response. In some cases, dendritic cells are dysregulated and their action can become impaired through pre-treatment or underlying disease. The T cell that is directed by the synthetic dendritic cell is not genetically modified allowing it to maintain its natural target identification, engagement and killing mechanisms.
NexImmune has two candidates in early stage clinical trials. This includes NEXI-001 for acute myeloid leukemia and NEXI-002 in multiple myeloma that are being advanced in the adoptive cell therapy modality. Other preclinical and discovery stage compounds fall into the injectable modality and include indications in solid tumors, autoimmune diseases and infectious diseases.
Exhibit III - Cross Section of Prominent Cell Therapy Companies[7]
| Company | Ticker | Price | Description | Headquarters | Market Cap |
|---|---|---|---|---|---|
| Capricor Therapeutics | CAPR | $4.16 | Cell & exosome-based therapeutics for the treatment of DMD using cardiac derived cells | Los Angeles, CA | $95.8 MM |
| Athersys, Inc. | ATHX | $1.29 | MultiStem platform using MAPC to treat stroke, trauma, HSCT & other diseases | Cleveland, OH | $291.9 MM |
| SQZ Biotechnologies | SQZ | $12.46 | Delivers cargo to cells by compressing cells in microfluidic channels for treating cancer | Watertown, MA | $349.5 MM |
| Adaptimmune Therapeutics plc | ADAP | $5.44 | Cell therapies for solid tumors using the SPEAR T cell platform. Lead ADP-A2M4 for synovial sarcoma | Abingdon, UK | $850.7 MM |
| Gamida Cell Ltd. | GMDA | $4.10 | Develops cell therapies to cure blood cancers and serious blood diseases | Jerusalem, Israel | $243.4 MM |
| NexImmune, Inc. | NEXI | $14.00 | Precision T cell technology | Gaithersburg, MD | $316.8 MM |
Summary
Cells make up the fundamental operating system of our body and allow us to perform all of our normal functions. In some cases our cells are defective or require an additional feature to improve our health, and cell therapy can address these difficulties and enhance a cell’s function.
Cell therapy has a long history of innovations and shows promise for addressing a multitude of diseases including cancer, HIV/AIDS, stroke, infectious disease and even chronic back pain. With well-known therapies such as CAR T and bone marrow transplants to less prominent Cell Squeeze approaches, the class of biologics can make substantial improvements in health navigated forward by the companies featured here.
[1] Cells can come from either autologous or allogeneic sources
[2] The first recorded successful blood transfusion to a patient who had hemorrhaged during childbirth, was performed by British physician Dr James Blundell in 1818.
[3] Which of course did not work.
[4] CRISPR - Clustered Regularly Interspaced Short Palindromic Repeats
[5] Source: SQZ Biotech Corporate Presentation, September 2021.
[6] Source: Adaptimmune website accessed October 2021.
[7] As of October 14, 2021





