Gene Therapy M&A: Best Prospects 2020

Contributing Author John Vandermosten, Senior Biotechnology Analyst
As we enter the twelfth year of an economic expansion, deal activity remains one of the primary ways for large companies to expand both the top and bottom line. One of the dominant industries in the merger and acquisition space has been biotech and life sciences. With scientific breakthroughs coming at an accelerating pace and the demand for new medicines expanding with an aging demographic, small and nimble companies have become attractive targets when they demonstrate a credible concept that advances medicine and can provide durable and cost-effective therapies.
After a decade long quiescent period, one area that has surged to the forefront of biotech is genomic medicine. Over the last year, there have been almost 30 biotech M&A deals, up from less than 20 in 2018. The increase was led by a number of high profile gene and cell therapy acquisitions in both the public and private spaces. This trend reflects the scientific advances that have been made over the last decades in making gene therapy a potentially permanent and safe approach to treating a broad variety of diseases.
Below we provide a summary of some of the genomic medicine deals that have taken place over the last couple years.
2018 Gene Therapy Deals
| Date | Acquiror | Target | Summary |
|---|---|---|---|
| 1/22/18 | Celgene | Juno | Cellular immunotherapy using autologous CAR T |
| 4/9/18 | Novartis | AveXis | AAV9 gene therapy for spinal muscular atrophy, Zolgensma |
| 8/10/18 | Astellas | Quethera | Recombinant AAV for use in glaucoma and other ophthalmologic |
| 9/20/18 | Amicus | Celenex | 10 clinical & pre-clin AAV programs in neurologic lysosomal storage disorders |
| 7/19/18 | PTC Tx | Agilis Bio | AAV Aromatic L-Amino Acid Decarboxylase Deficiency |
2019 Gene Therapy Deals
| Date | Acquiror | Target | Summary |
|---|---|---|---|
| 2/25/19 | Roche | Spark Tx | AAV gene therapies in blindness, hemophilia, lysosomal storages disorders, neurodegenerative diseases |
| 2/27/19 | Sarepta | Myonexus | AAVrh.74 Five gene therapy candidates to treat forms of Limb-Girdle Muscular Dystrophy. 3 clinical, 2 preclin assets |
| 3/4/19 | Biogen | Nightstar | AAV clinical stage gene therapy. AAV treatments for retinal disorders choroidemia |
| 9/12/19 | Castle Creek | Fibrocell | Autologous LV cell & gene therapy for skin & connective tissue disease. Ph3 for recessive dystrophic epidermolysis bullosa |
| 12/2/19 | Astellas | Audentes | AAV based genetic medicines. X-linked myotubular myopathy, Pompe and Duchenne muscular dystrophy |
What is Genomic Medicine?
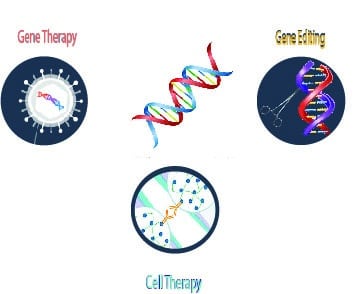
There are three main technologies in genomic medicine which include gene therapy, cell therapy and gene editing. Gene therapy replaces defective genes related to an inherited or acquired disease. It delivers a working copy of a gene to the target cell using a vector such as adeno-associated virus (AAV), lentivirus (LV) or other viral vector. Cell therapy is the transfer of allogenic or autologous cells into a patient to treat a disease. A subset of this approach is genetic cell therapy where a cell is removed, modified and returned to the body such as the Yescarta and Kymriah therapies for blood cancers. Gene editing uses molecular scissors to cut, remove and replace a sequence of DNA. Some of the better known gene editing approaches include CRISPR-Cas9, ZFN and TALENS. 1 As of early 2020, the categories of gene therapy and cell therapy have seen multiple approvals while gene editing remains in the clinic.
Graphic by Darreah Wilson
Gene Therapy Backgrounder
The launch of interest into gene therapy was thrust into the mainstream scientific world with a 1972 paper entitled “Gene Therapy for Human Genetic Disease?” authored by Theodore Friedmann and Richard Roblin. While it started out with a warning, it also highlighted the immense potential of the approach. An early success treating severe combined immunodeficiency disease (SCID) in four year old Ashanti DeSilva was followed by a later failures resulting in the deaths of Jesse Gelsinger and leukemia in several children receiving treatment for SCID-X1. Following these unfortunate events, interest in the area of gene therapy evaporated. There was some approval activity in China and Russia in the intervening years, but it wasn’t until 2012 when a gene therapy drug was first approved in the West. The drug that finally broke through was UniQure’s Glybera which was given the go-ahead by the European Commission to treat lipoprotein lipase deficiency in October of that year. While it was found safe and effective by regulatory authorities, the product failed to gain traction, largely due to its €1 million price tag.
Since then, other approvals have taken place in the space. Ex-vivo cell therapy treatments for blood cancers Kymriah and Yescarta were approved by US regulatory authorities in fall of 2017 and Spark’s treatment for a rare type of blindness, Luxturna, took place in December of 2017. After a break of almost a year and a half, the U.S. Food and Drug Administration (FDA) got back to approving gene therapy treatments with a nod towards AveXis’ Zolgensma for spinal muscular atrophy in May 2019.

These approvals came on the heels of advances made in virus biology, vector dynamics, immune interaction and vector safety eliminating many of the risks present in earlier approaches. As the science has advanced, so has the desire for larger competitors to bring this technology in-house and leverage the anticipated benefits of the technology.
Gene Therapy Backgrounder
 As many of the risks have been addressed, the regulatory agencies have also become more familiar with the perils and potential of gene and cell therapy, we anticipate that interest in the space will accelerate. There are over 1000 different types of gene therapies in clinical trials according to the American Society of Gene & Cell Therapy. With the science advancing, an expanding pipeline and the FDA and EMA on board with the technology, we see a backdrop that is favorable to an accelerating number of deals.
As many of the risks have been addressed, the regulatory agencies have also become more familiar with the perils and potential of gene and cell therapy, we anticipate that interest in the space will accelerate. There are over 1000 different types of gene therapies in clinical trials according to the American Society of Gene & Cell Therapy. With the science advancing, an expanding pipeline and the FDA and EMA on board with the technology, we see a backdrop that is favorable to an accelerating number of deals.
While there are some hurdles to overcome in 2020, including a presidential election, coronavirus, healthy valuations and concerns over drug pricing, there are also many reasons to see further transactions. The top 15 biopharma companies have a combined $171 billion in cash and borrowing is relatively cheap. Bristol Myers Squibb, for example holds over $32 billion in cash and maintains an interest in gene therapy while others such as Biogen (NASDAQ: BIIB), Gilead (NASDAQ: GILD) and Sanofi (NASDAQ: SNY) all have reasons to find attractive partners and acquisitions in genomic medicine.
Genomic Medicine M&A Targets
A number of gene therapy companies may be the next targets in the race to find the next growth vector in this space. A few companies that stand out as companies to watch include Sarepta Therapeutics (NASDAQ: SRPT), BioMarin (NASDAQ: BMRN), bluebird bio (NASDAQ: BLUE) and American Gene Technologies (AGT).
Sarepta Therapeutics has been presumed to be an M&A target for several years now. The company is focused on Duchenne muscular dystrophy, an inherited disorder that affects muscle strength. Sarepta has two commercial drugs for Duchenne using RNA technologies and two others in clinical development for the same disease in gene therapy. In total, the company has eight clinical assets and many more in earlier stages. With a market cap above $9 billion, the company is more than a small bite but provides three modalities in genomic medicine that could bulk up the portfolios of some of the big names in the space such as Roche (OTC: RHHBY) or Biogen whose future in the neurodegenerative space is uncertain.
BioMarin Pharmaceuticals is an even bigger mouthful for a major with an almost $17 billion market cap, however the middleweight gene therapy with seven commercialized orphan drugs has a broad portfolio of assets including three gene therapy drugs. The most advanced is Valoctocogene Roxaparvovec for Hemophilia A which could be approved this summer. Biomarin has a partnership with Genzyme, a subsidiary of Sanofi which may want to up its stake in the idea-rich biotech. Biogen, Roche and Gilead also have strong balance sheets, a need for growth and interests in gene therapy, making them potential suitors.
bluebird bio boasts a recently approved lentiviral vector delivered gene therapy for β-thalassemia designated Zynteglo. The development pipeline is impressive with 14 clinical stage product candidates in severe genetic diseases and oncology. Share price is well off of highs and the diverse long tail pipeline make it attractive for acquirers that can benefit from both current and future revenues.
And even American Gene Technologies,2 a private company, which is expected to go into the clinic in the first half of 2020, may be attractive to gene therapy buyers. The company is working on a cure for HIV. The therapy, known as AGT103-T, requires patients to be stable on antiretroviral therapy (ART) medications before they can qualify for the gene therapy. While many with HIV avoid taking the medicines in the early stage of the disease due to cost and side effects, the possibility of a cure would drive substantial demand. A company such as Gilead with over 10 products targeting this market could synergize substantially with AGT and experience a dramatic near term surge in the demand for their ART. If AGT’s clinical trials demonstrate promise, we may see action from Gilead.
And In Closing
The environment for genomic medicine M&A appears to be favorable with advances in science, regulatory receptiveness to the class, plenty of cash on big pharma balance sheets and a need to add growth opportunities to their portfolio. With ten deals over the last two years, there appears to be an appetite for more.
1 CRISPR: Clustered regularly interspaced short palindromic repeats; ZFN: Zinc finger nuclease; TALEN: transcription activator-life effector-based nucleases.
2 Disclosure: Author has received compensation from one or more of the companies mentioned in this article.





