Gene Therapy Clinical Trial Requirements


Contributing Author John Vandermosten, Senior Biotechnology Analyst
Gene Therapy Does It “My Way”
In 1990, gene therapy burst onto the world stage with the first clinical trial aimed at a genetic disease. This effort, led by a team at the National Institute of Health, successfully treated a four-year-old girl with severe combined immunodeficiency (SCID), more commonly known as bubble boy disease. The trial’s structure was significantly different from the traditional approach: rather than establishing safety in healthy adults, the FDA-authorized trial pursued a cure by directly treating two pediatric patients suffering from this serious genetic condition. So what is it about gene therapy that permits such a direct approach?
In this article we will review the phases of traditional clinical trials that lead to FDA approval, then describe what features distinguish trials in gene therapy. We’ll highlight some unique features of the American Gene Technologies® Phase I RePAIR trial, which aims to functionally cure HIV. Then we’ll explore gene therapy’s use in pediatric patients for inherited diseases.
The Traditional Medicine Clinical Trial Progression
In 1990, gene therapy burst onto the world stage with the first clinical trial aimed at a genetic disease. This effort, led by a team at the National Institute of Health, successfully treated a four-year-old girl with severe combined immunodeficiency (SCID), more commonly known as bubble boy disease. The trial’s structure was significantly different from the traditional approach: rather than establishing safety in healthy adults, the FDA-authorized trial pursued a cure by directly treating two pediatric patients suffering from this serious genetic condition. So what is it about gene therapy that permits such a direct approach?
In this article we will review the phases of traditional clinical trials that lead to FDA approval, then describe what features distinguish trials in gene therapy. We’ll highlight some unique features of the American Gene Technologies® Phase I RePAIR trial, which aims to functionally cure HIV. Then we’ll explore gene therapy’s use in pediatric patients for inherited diseases.
Drug Development Process1
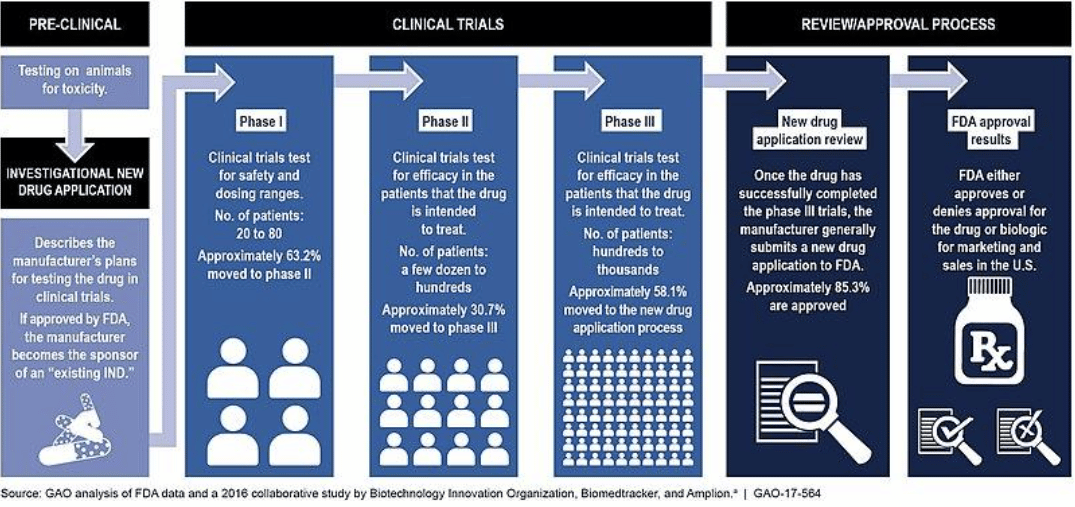
Most INDs submitted by large sponsors are designated as Commercial INDs. These are intended for experimental drugs showing promise in clinical testing for serious or immediately life-threatening conditions. The IND will include data containing animal pharmacology and toxicology studies, manufacturing information, clinical protocols and investigator information. After submission, the FDA has 30 days to review and either request further information or clear the drug product for in-human trials.
Phase I
Traditionally, clinical trials begin with a Phase I study that enrolls healthy volunteers to evaluate the safety of a new drug or therapy. The goal of this early clinical work is to establish if a new treatment is safe, determine the best method of administration and identify the optimal dose. By using healthy volunteers, the impact of disease will not interfere with the measurement of drug toxicity, yielding a more accurate safety profile for the new drug. If the product has an acceptable side effect profile, then it is advanced to Phase II.
Phase II
Phase II trials continue to focus on safety but also evaluate the treatment’s performance in individuals affected by the target condition. This stage attempts to ascertain drug efficacy by performing a randomized, double-blind, controlled study. Phase II studies usually enroll more patients and study them for a longer period of time as compared to previous phases, to support proof-of-concept. If the results are favorable and show a statistically significant improvement while maintaining an acceptable degree of safety, the drug candidate is then advanced to Phase III.
Phase III
A Phase III study perseveres with the emphasis on safety and efficacy, but raises the standards compared to a Phase II by increasing the size and refining the endpoints required for approval. A drug in Phase III can be under investigation for many years and potentially enroll thousands of subjects in a randomized, double-blind, placebo-controlled trial. The trial is divided into a control group that receives either a placebo or standard of care and an active group that receives the new treatment.
Gene Therapy Clinical Trials
Gene therapies behave differently in the body compared to traditional medicines, and clinical trial design reflects this. Generally, gene therapy trials will only include subjects with the target condition since these therapies often aim to change the behavior of long-lived cells, creating a permanent or semi-permanent effect. In some cases, the patient’s own cells are taken out of the body, modified in the lab, then re-introduced, adding further complexity and safety considerations. In other cases, gene therapy is used to intervene in pediatric patients whose condition may be lethal or cause developmental problems if left untreated; presenting further safety and efficacy considerations. Gene therapy trial design may also add efficacy metrics as endpoints in Phase I studies.
Early Stage Trial Distinctions
Phase I studies are designed to accommodate the unique set of risks involved with testing a gene therapy. For example, some therapeutic approaches may modify long-lived cell populations, making it unethical to administer to healthy individuals. Additionally, certain therapeutics may require certain immunological conditions to function correctly; in the case of American Gene Technologies’ lead candidate, AGT103-T, only T cells which recognize pieces of HIV are modified, so without the presence of HIV, the cells are not stimulated. For therapeutic approaches which utilize immunogenic viral vectors, repeat dosing may also be dangerous because the immune system may remember certain viral vectors2 and react with increasing vigor upon repeat dosage. Well-designed Phase I trials account for therapy-specific factors as well as general safety metrics to provide a complete safety and side-effect profile.
As it can make permanent changes inside a cell, gene therapy is a fundamentally different therapeutic modality from large and small molecule medicine. The potential for prolonged biological activity after a single administration requires long-term follow up. As a result, the FDA asks sponsors to follow up with patients for 15 years after dosing a variety of viral vectors and genome editing products3. This checkup allows researchers and regulators to understand how the gene therapy functions long-term, verify the reliable and controlled expression of the delivered gene, and ensure that the gene therapy does not interfere with the normal function of a critical enzyme, hormone, or biological process in the recipient. Genetic misregulation can cause problems under certain conditions, so gene therapy trials are designed with special monitoring considerations to identify any potential for carcinogenesis, acute or delayed infusion reactions, autoimmunity, graft failure, transmission of infectious agents from a donor and latent viral reactivation.
Autologous Cells and Trial Design
At a base level, gene therapy installs functional modifications into cells, so the survival and ability of these cells to perform their intended function is essential to efficacy endpoints. Later-stage trials can differ in design based on the source of the cells and methods of introducing the therapeutic modifications. For instance, drug products using autologous tissue are created in the lab when patient-derived cells are modified. In many trials, these patient-derived cells are blood cells collected through apheresis4. Researchers can perform safety and functionality checks on the modified cells before they are re-introduced to the body, or even run preclinical efficacy screenings in the lab to show regulators how the cells work in vitro before clinical trials even begin. AGT103-T was tested in this way before the Phase I trial, giving a strong indication for its in vivo potential. Meanwhile, therapeutics which use an in vivo modification may not be able to measure efficacy in human cell systems before trials begin, relying on animal models to justify first human use. These variables can complicate the trial design, the timing of treatment and the analysis of results.
Apheresis for Gene Therapy5
Pediatric Use
Gene therapy is particularly effective against genetic disease, and oftentimes, these disorders manifest early in life and disrupt critical developmental pathways, causing permanent damage or death. Treating pediatric subjects as opposed to adults may be more appropriate, as a cure will provide life-long benefits and can prevent the accumulation of permanent damage. In cases where pediatric intervention is necessary, additional caution is required which carries significant trial design implications as the physiology of pediatric patients changes with development. Only children that are seriously ill or have a disease that cannot be cured using standard of care can receive gene therapies.
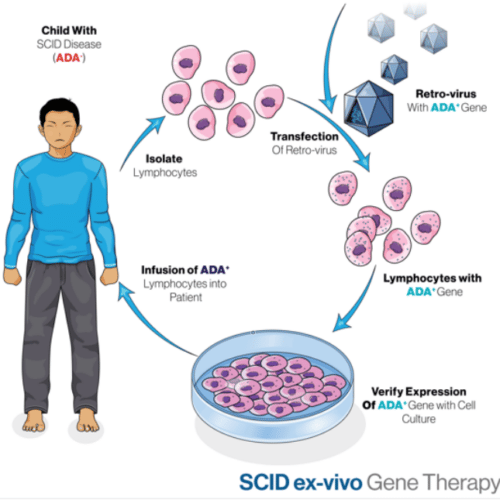
In some cases, such as for primary immune deficiencies, Rett syndrome or Tay-Sachs disease, the children afflicted will likely not survive to adulthood and only gene therapy treatment can cure them. Treating diseases early in kids can prevent permanent harm and might be the only window where gene therapy can be effective. A UCLA study that delivered gene-corrected cells to 10 children with ADA-SCID found that the treatment was durable in 9 of the 10 patients. Most of the children were babies. The oldest child, who was 15 at the time of the treatment, was the only one whose immune function was not restored.
Gene Therapy Treatment for SCID6
Trial Design Differences
Trial design for gene therapy is distinct from that used for small and large molecules. For example, Phase I studies in gene therapy look not only at safety, but also consider feasibility of administration and pharmacologic activity. Some Phase I studies include selected features of later stage studies to gather preliminary evidence of effectiveness.
While all early human studies seek to determine the candidate’s activity, the goals of early-stage trials are broader for gene therapy. These efforts include specialized metrics such as gene expression, cell engraftment, and morphologic alterations on top of standard metrics, including changes in immune function or physiologic responses of various types. For example, in American Gene Technologies’ RePAIR study, trial participants were enrolled and dosed in a staggered fashion to ensure that the first patient suffered no negative effects from AGT103-T prior to the second one being dosed. The study was designed to measure critical safety and treatment-related metrics such as cytokines in the blood and biomarkers indicative of cell engraftment. Another prominent feature of RePAIR that distinguishes it from traditional clinical trials is the analytic treatment interruption (ATI) study, where participants were withdrawn from antiretroviral medications to observe results under different immunological conditions.
Other Concerns
Patients that receive gene therapy require observation to guard against delayed infusion reactions. Some of the conditions monitored include autoimmunity, graft failure, graft vs. host disease (GVHD), transmission of infectious agents from a donor and viral reactivation. Another concern specific to gene therapy is viral or vector shedding. This phenomenon relates to how the product is excreted from the patient’s body. While most viral vectors are replication-deficient and cannot copy themselves, other viral interventions such as oncolytic viruses used for cancer treatment, may be able to multiply. In the latter category, there may be a possibility that the shed vector may be infectious and investigation is necessary to understand the potential risk.
Charting a Different Course
Gene therapy has emerged as an effective method to treat genetic and other disease that can be corrected with genetic modification. It has presented possibilities for curing many diseases, which up until now, could only be addressed with palliative care. Given gene therapy’s unique mode of action, there are many reasons why the traditional approach to clinical trials should be modified to reflect its specific risks and opportunities.
The traditional approach to clinical trials fails to address the permanent nature and altered risk profile of gene therapy. In the past, the focus was first safety, then a methodical advance forward with increasing trial size to also evaluate efficacy. The more powerful tool of gene therapy requires dosing patients with the targeted condition to determine its safety profile. And since many gene therapies address genetic disease present at birth, beginning with the youngest patients is most reasonable. Gene therapy may also present long-term implications due to the life expectancy of the cell type that researchers choose to modify. The enduring impact requires extended follow up for gene therapy patients, but also presents the potential for long-term benefits from a single dose.
Gene therapy is fundamentally different from the action of small and large molecule drugs, and clinical trials have adapted to effectively measure its safety and efficacy. It is an exceptional tool that offers a distinctive risk profile and the potential to provide cures for previously incurable diseases. Some gene therapy trials, such as American Gene’s RePAIR, embody many of the features we discuss, providing an excellent example of the unique nature of gene therapy trials.
1 GAO analysis of FDA data, Biotechnology Innovation Organization, Biomedtracker and Amplion.
2 Viral vectors are viral shells that are used to deliver the desired genes.
3 Long Term Follow-Up After Administration of Human Gene Therapy Products. FDA Industry Guidance, January 2020.
4 Apheresis is the removal of blood plasma from a patient by the withdrawal of blood, its separation into plasma and cells, and the return of the cells to the patient’s bloodstream.
5 Source: Shutterstock Image 1252254298
6 Source: Shutterstock Image 1959146710





