Chapter IV: Solving The Riddle, A Consequential Approach


Contributing Author John Vandermosten, Senior Biotechnology Analyst
The arc of HIV history has been developing over the last four decades. In the early days there was much we did not know, but as teams of physicians, researchers and patients increased their understanding of the disease, progress was made. Early efforts with repurposed cancer drugs gave way to numerous viral inhibitor combinations. On a parallel course, attempts to develop a vaccine have been underway since 1984, but with limited success. A cure has also been elusive, with only a few individuals achieving complete suppression of HIV as a peripheral benefit to hematopoietic stem cell replacement therapy for blood cancers. A new hope has emerged in gene therapy, and one of the most promising approaches is AGT™lead candidate AGT103-T which recently entered clinical trials and infused its first patient. We discuss the background of AGT103-T and other recent efforts to combat HIV in our fourth installment of the Arc of HIV History.
A Cold Start
It was a snowy day in early 2007, and former tech executive Jeff Galvin exited his airport shuttle, walking towards the National Institutes of Health Building in Bethesda, Maryland. He was scheduled to meet with Dr. Roscoe Brady, an eminent recipient of the Lasker Award and developer of the first enzyme replacement therapy for Gaucher Disease. This meeting was one of several that Jeff had arranged in his search for a significant scalable opportunity that could benefit from his background in the tech industry. Although not evident at the time, it was to be an auspicious day that would place events in motion leading to our best shot yet at a cure for HIV.
Exhibit I - National Institutes of Health1

During the meeting, Jeff was introduced to Dr. Brady’s portfolio of lentiviruses and plasmids that serve as vehicles for gene therapy. Dr. Brady had spent decades developing enzyme replacement therapies to treat lysosome storage disorders such as Fabry and Gaucher disease. He believed that the approach could be used to cure, rather than just treat, these lysosomal maladies, helping free patients from a never-ending drug regimen. Dr. Brady’s goal was to replace the broken gene that coded for the missing enzyme, leading to a durable solution for these diseases, emancipating these patients from lifelong treatment.
After learning of Dr. Brady’s accomplishments and understanding the potential of his lentiviral vectors, Jeff assumed the role as standard bearer for this work. His vision for the future of the industry incorporated the replicable and iterative nature of this new software industry for the code that makes us human. Inspired by Dr. Brady’s friendship and encouragement, he founded American Gene Technologies® and began the years-long effort to build a team that could use these viral vectors to treat a disease of consequence.
The Evolution in HIV Treatment
As AGT’s senior scientists performed the validation work characteristic of the early stages of drug development, antiretroviral therapy (ART) agents were evolving. In 2007, two products called Maraviroc and Raltegravir were approved. The first was a CCR5 antagonist which blocked CCR5 receptors on CD4+ cell surfaces thereby preventing HIV entrance. The second was an integrase inhibitor, which arrested the enzyme necessary for the integration of viral DNA into host cells. While these were improvements, the formulations continued to only treat the disease rather than cure it.
During the first decade and a half of the 21st century, the magnitude of improvements in HIV treatment was slowing even though progress was being made. In 2012, the FDA approved pre-exposure prophylaxis (PrEP) for HIV-negative individuals to prevent sexual transmission of HIV. Also in 2012, for the first time in history, the majority of those eligible for HIV treatment were receiving it.2 A number of new combinations of HIV treatments were approved in 2018; however, these were primarily previously recognized compounds that had been patched together to reduce the number of pills a patient had to take. There were also indication expansions approved during and after 2018. Pregnant women, adolescents and pediatric patients were all cleared to use existing medicines and preventative use of ARTs, such as PrEP, was added to the FDA’s Orange Book. New approaches were introduced including Trogarzo (2018) and Rukobia (2020) intended for patients failing prescribed therapy.3 Overcoming resistance was becoming increasingly important, combating a trend that was confirmed in a Lancet article4 which found that HIV resistance to tenofovir was becoming increasingly common.
Exhibit II – Global HIV Statistics (in thousands)5
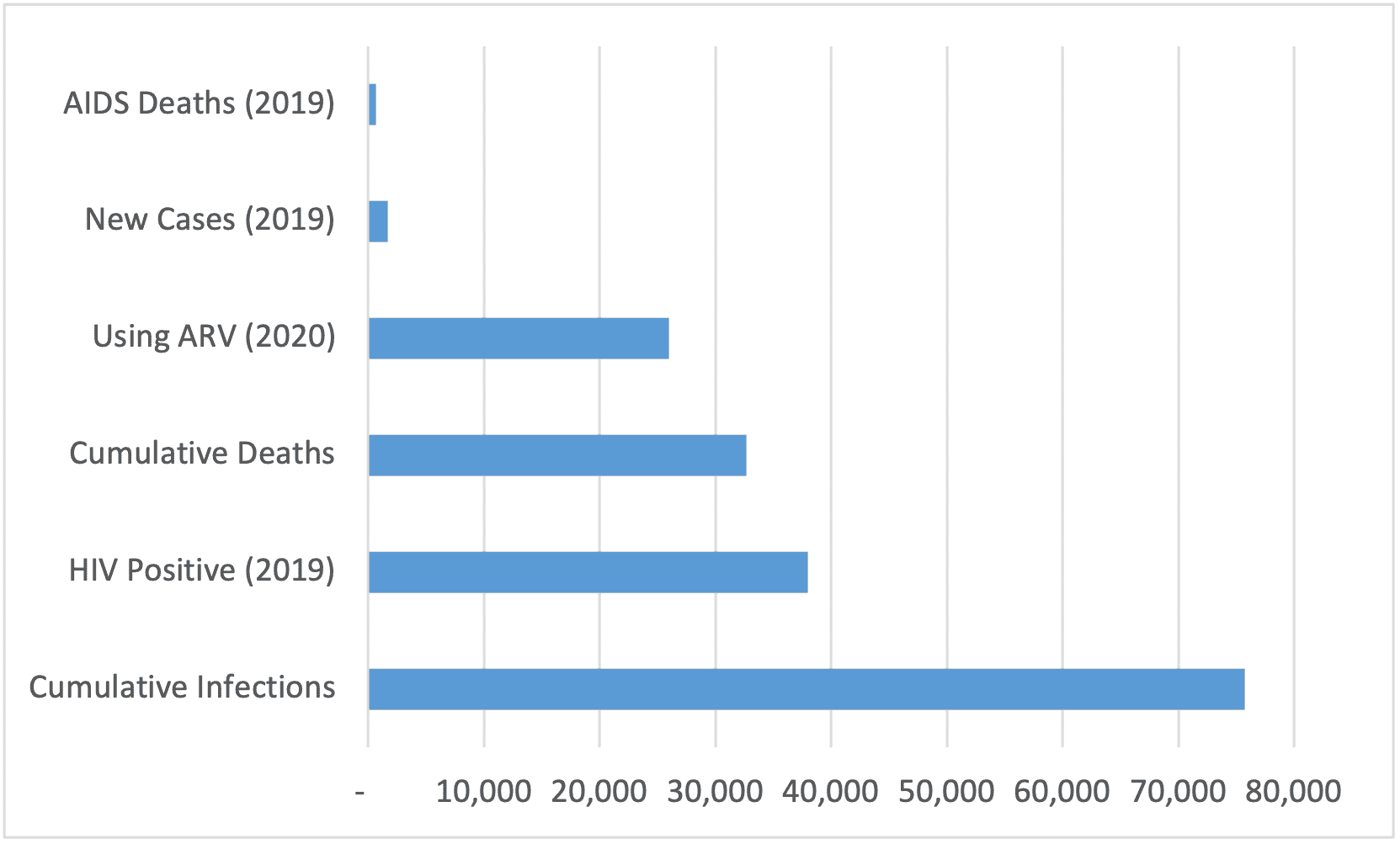
An Ounce of Prevention…
Parallel with the efforts to develop treatments was work to create immunity to HIV through a vaccine. The creation of such an immunization that can protect people from HIV has been an ongoing mission since the early days of HIV’s widespread emergence. The first efforts to develop a vaccine were launched in the 1980s with a Phase I trial initiated at the NIH in Bethesda, Maryland. The first large-scale HIV study was begun in 1998 by VaxGen which launched a Phase III trial in the US and Europe. In 2000, the HIV Vaccine Trials Network (HVTN) was formed by the National Institute of Allergy and Infectious Diseases (NIAID) and was awarded a grant from the National Institute of Health to integrate clinical development of a vaccine. Its role has been to identify HIV vaccine candidates and according to its website “has conducted the majority of the published, presented, or ongoing clinical trials of preventive HIV vaccines worldwide.” Despite many clinical attempts with HIV vaccine candidates, none have been approved. Variability of HIV types, frequent viral mutation, absence of natural immunity and the lack of an effective animal model are faulted for the dearth of progress.
The best performance of a vaccine to date has been the RV144 prime-boost combination comprised of the ALVAC HIV vaccine and AIDSVAX B/E vaccine. A trial conducted in Thailand used this combination to reduce HIV infection by 31% compared to placebo as described in a New England Journal of Medicine article. However, the vaccine was not able to achieve the accepted hurdle of 50% effectiveness that is commonly reached before applying for approval. The search continues with two trials designated Imbokodo and Mosaico now underway which are predominantly being conducted in Africa and Latin America respectively. Janssen, which is running both of the studies, is expected to provide readouts by 2021.
The Revolution in HIV Treatment
While the advancement of new drugs in the HIV field made stepwise improvements, the absence of progress developing a vaccine and a practical cure continues to elude scientists, physicians and patients. We turn the focus back to AGT, which has the end in mind using a modular approach to harden the body’s immune system against HIV and prevent existing HIV from propagating. To get there, AGT needed an expert with a deep background in both virology and gene therapy who could inspire the team to go beyond what other innovators such as Sangamo and Calimmune had achieved.
In 2011, Dr. David Pauza began to work with AGT full time. He had been tirelessly engaged with viral diseases for decades prior to his work with the company. Following his Ph.D. at U.C. Berkeley and postdoctoral work in Cambridge, England he initiated the HIV/AIDS research program at the Salk Institute for Biological Studies in La Jolla, California in 1985. Dr. Pauza later moved to the University of Wisconsin-Madison to build another HIV/AIDS program. This background made him the perfect match for AGT’s efforts in developing a gene therapy cure for HIV.
Exhibit IV - AGT Offices 15010 Broschart Road (2011 – 2017)

As the status quo was gradually evolving treatments for HIV, AGT was laying the groundwork for a revolution in HIV treatment. CEO Jeff Galvin’s background in technology saw gene therapy from a different perspective. Gene therapy was a way to modify the human program which was coded in DNA. If the code has a bug in it, then gene therapy could fix it. HIV had been an elusive target for a cure, and AGT, recognizing the competitive advantages of its gene therapy platform, decided to take a shot at it.
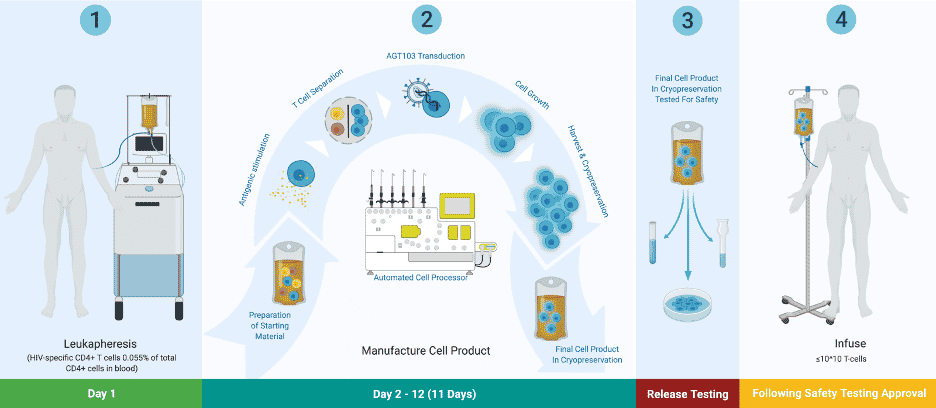
Others had tried to replicate the process that had worked in the successful stem cell transplants to eradicate HIV, but had not been able to crack the code. AGT recognized the flaws in the previous efforts and designed its approach to create a durable, HIV-specific immune response by culturing a large and diverse enough population of HIV specific helper T cells to reconstitute the normal immune response against the virus. To protect the new population from being co-opted, genes are inserted that delete the CCR5 co-receptor and generate permanently expressing siRNA that neutralize viral proteins necessary for HIV replication.
After years of work to perfect the approach, AGT finally had something that could succeed in the clinic. The approach used here reinvigorates and supports a potent antiviral immune response to HIV by reconstituting Gag-specific CD4+ T cells and engineering these same cells to resist HIV infection and inhibit HIV release from latently infected cells. The immunotherapy additionally restores the capacity for generating cytotoxic CD8 T cells and neutralizing antibodies. In the clinic, AGT will be able to test if having a sufficient number of Gag-specific CD4+ (helper) T cells can purge HIV over time.
Exhibit V – AGT103-T HIV Immunotherapy6
In-Human Trials
After years of early development work, in 2016, AGT met with the FDA to navigate a path forward to use AGT103-T in HIV patients. Close contact with the agency provided guidance for conducting the preclinical work required to gain clearance to start clinical studies. After three years of effort, AGT placed the final touches on its investigational new drug application (IND) to begin in-human trials and submitted the document to the FDA in October 2019.
To date, relatively few gene therapy trials have been cleared by the agency, and regulators were determined to clarify any perceived risks to new technologies. This stance led to several months of additional questions and responses between the two parties, leading to eventual clearance of the IND to investigate AGT103-T in August 2020. After a short delay, which was required due to the need for more testing, the trial enrolled its first patient in November 2020 at Washington Health Institute, University of Maryland, Institute of Human Virology and Georgetown University trial sites. The Phase I trial will investigate the safety of AGT103-T, measure key biomarkers and explore surrogate markers of efficacy. The company is currently designing an analytical treatment interruption protocol to assess antiretroviral therapy discontinuation after a participant receives the treatment. The first patient was infused in May 2021 and returned home the same day. We may soon know if the first patient was cured of HIV using gene therapy.
AGT103-T
AGT103-T is a genetically modified cell product made from a patient’s own cells. The AGT approach is unique in its emphasis on repairing immune system damage caused by HIV by enriching for HIV-specific CD4+ T cells in culture, rendering cells immune to further HIV infection, and suppressing HIV replication using permanently expressing siRNA. If effective, the drug candidate is a one and done functional cure for HIV. A research paper published in Molecular Therapy entitled “Preclinical Development and Clinical-Scale Manufacturing of HIV Gag-Specific, Lentivirus Modified CD4 T Cells for HIV Functional Cure” discusses the therapy and process used in detail. A readout of the trial is expected later this year providing the data necessary to move into the next phase of clinical trials which will further examine the efficacy and safety of this innovative gene therapy.
Exhibit VI – AGT’s Rockville, Maryland Facility, 2021

While it will take many months to be confident that AGT103-T was able to produce a functional cure it is possible that the first HIV patient has already been cured using this drug. AGT will provide press releases for the duration of the trial updating stakeholders on its progress.
Summary
Since HIV emerged into the public consciousness forty years ago, much progress and learning about the virus have occurred. Antiretrovirals have made it possible to suppress HIV to near undetectable levels and in rare cases, patients with blood cancers receiving hematopoietic stem cell transplants may fully clear the disease. While ARTs, vaccines and stem cell transplants have distinct shortcomings, new approaches may present a better alternative. In response, AGT is building on the shoulders of giants by drawing on the elements of gene therapy and combining them in a unique way to target the weaknesses of HIV. The effort has made many advances in finding a cure for this pernicious disease and may deliver the next milestone just ahead.
Sources:
1https://news.colgate.edu/wp-content/uploads/2014/01/NIHexterior.jpg
2UNAIDS 2012 Global Report.
3 https://www.uspharmacist.com/article/newly-approved-hiv-medications
4TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. The Lancet. January 28, 2016 https://doi.org/10.1016/S1473-3099(15)00536-8
5Author compilation of data from UNAIDS Fact Sheet – World AIDS Day 2020.
6Source: American Gene Technologies





