American Gene Technologies to Present at 2020 Inborn Errors of Metabolism Drug Development Summit in Boston

ROCKVILLE, Md., March 10, 2020 (GLOBE NEWSWIRE) -- American Gene Technologies (AGT), a leading gene and cell therapy company in Rockville, Maryland, is pleased to announce its Chief Science Officer C. David Pauza, Ph.D will present at the Inborn Errors of Metabolism Drug Development Summit in Boston, MA on March 11, 2020.
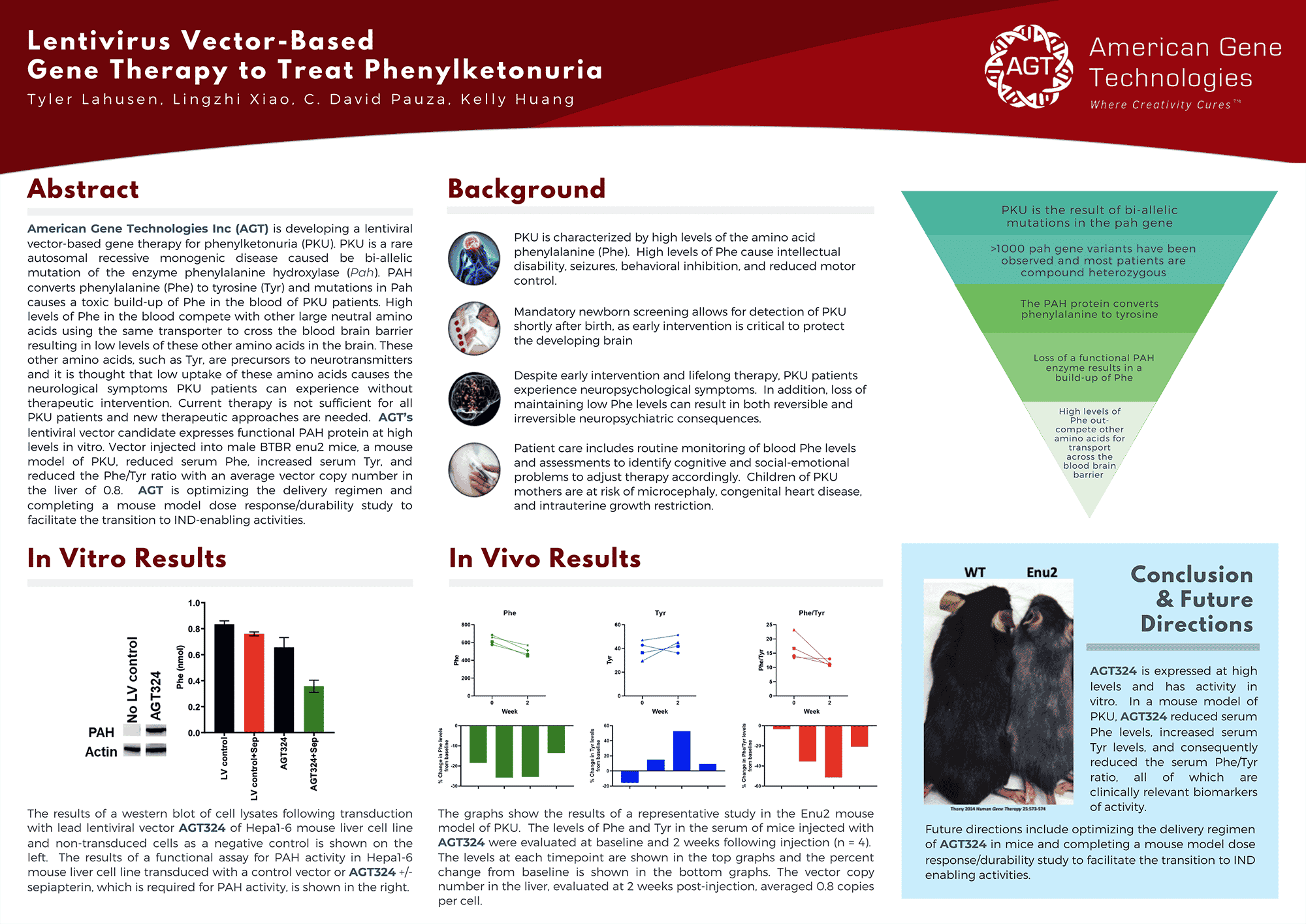
Dr. Pauza will present how AGT used its proprietary, lentiviral vector based technology to develop a treatment for phenylketonuria (PKU), a debilitating inherited disease affecting 1 in 13,500 children born in the U.S. AGT's lentiviral vector approach is intended to provide a permanent cure with the goal of improving quality of life for more than 16,000 people living with PKU in the U.S. alone. This treatment is currently in pre-clinical development and has received orphan-drug designation #DRU-2018-6572 by the U.S. Food and Drug Administration (FDA) (press release).
More specifically, Dr. Pauza’s presentation, “Treating PKU by Lentiviral Vector Gene Delivery to Liver,” will cover AGT’s PKU asset. Key topic areas will include:
- Exploiting natural tropism of lentivirus vectors
- Permanent genetic modification suitable for infants
- New strategies to improve hepatocyte transduction
Details of his conference presentation:
| Event: | 2020 Inborn Errors of Metabolism Drug Development Summit |
| Speaker: | C. David Pauza, Ph.D., Chief Science Officer at AGT |
| Date: | Wednesday, March 11, 2020 |
| Time: | 12:30 a.m. EDT |
| Location: | Courtyard Boston Downtown/North Station, 107 Beverly St., Boston, MA |
Dr. Pauza will be joined at the conference by two other members of the American Gene Technologies team: Irene Tennant, M.Sc., VP of Clinical Product Development and Regulatory Affairs and Kelly Huang, Ph.D., Director of Preclinical Product Development.
Dr. Kelly Huang will present a poster on American Gene Technologies’ Phenylketonuria (PKU) asset AGT324 during the poster session of the conference. Download the poster HERE.
About Speaker C. David Pauza, Ph.D., Chief Science Officer, AGT:
C. David Pauza, PhD is Chief Science Officer for American Gene Technologies and Adjunct Professor of Medicine at the University of Maryland Medical School in Baltimore. Prior to joining AGT, Dr. Pauza was Associate Director for the university’s prestigious Institute of Human Virology and Co-Leader of the Greenebaum Cancer Center Program in Viral Oncology. He is an internationally recognized expert in human virology and viral diseases including HIV, arenaviruses, poxviruses and herpesviruses. He has published more than 150 scientific papers and holds twelve U.S. patents.
About Kelly Huang, Ph.D., Preclinical Product Development, AGT:
Kelly Huang, PhD is Director of Preclinical Product Development for American Gene Technologies. Dr. Huang is a scientist with extensive experience developing cell-based and molecular-based assays, as well as, building ground-up research programs for both virology and human immunology. Kelly has led preclinical and translational research efforts to enable clinical stage drug development. Prior to joining AGT, Dr. Huang was the Scientific Director at Precision for Medicine where she built a team of scientists that developed a variety of drugs in oncology, autoimmunity and infectious disease.
About Irene Tennant, M.Sc., Clinical Product Development and Regulatory Affairs, AGT:
Irene Tennant is the VP of Clinical Product Development and Regulatory Affairs. She has more than 20 years of experience in clinical research & development and operations management for leading pharmaceutical and biotechnology companies, including Pfizer and Novartis. She has successfully built and led clinical teams supporting the development of first in class treatments in gene medicine, oncology, and infectious diseases. Since 2005, Irene has supported the development of cell and gene therapy treatments to address solid tumor cancers and degenerative eye diseases.
About American Gene Technologies (AGT)
American Gene Technologies (AGT) is a gene and cell therapy company with a proprietary gene-delivery platform for rapid development of cell and gene therapies to cure infectious diseases, cancers, and inherited disorders. The Company's mission is to transform people's lives through genetic medicines that rid the body of disease. The Company expects to take its patented lead candidate for an HIV cure to the clinic in 2020. AGT has an extensive patent and intellectual property portfolio that includes infectious disease, monogenic disorders, and cancer, including seven patents for its unique immuno-oncology approach to stimulate gamma-delta (γδ) T cells to destroy a variety of solid tumors. The Company has developed a synthetic gene for treating Phenylketonuria (PKU), a debilitating inherited disease. AGT's treatment for PKU has been granted Orphan Drug Designation by the Food and Drug Administration (FDA), and it is expected to reach the clinic in 2020.
To learn more about AGT’s PKU treatment and/or partnership opportunities, please contact us here.
About the Inborn Errors of Metabolism Drug Development Summit:
Inborn Errors of Metabolism Drug Development Summit is a three-day conference that brings together over 80 inherited metabolic disease drug developers and key opinion leaders from the likes of Takeda, Sanofi, BioMarin and Regeneron. The Summit is the first and only meeting dedicated exclusively to solving companies’ translational drug development challenges. This end-to-end conference will provide the platform, resources, and support for companies to overcome their drug development hurdles by furthering their understanding of disease progression and therapeutic outcomes, developing more robust and effective therapeutic delivery systems, and improving their knowledge of the payer and reimbursement landscape. The conference features 24 expert speakers, workshops and a poster session.
For a full agenda, click here. For questions about the event, contact Stephanie Affleck, Event Coordinator Stephanie.Affleck@hansonwade.com, or Fergus Cunningham, Program Director Fergus.Cunningham@hansonwade.com.
A poster accompanying this announcement is available HERE.