NEWSLETTER VOLUME 9

FDA Progress
The Path to the Clinic
For the HIV Cure
This month, AGT saw tremendous progress with the FDA. FDA time and attention to our project has seemed to increase while agency review responses to our submissions have narrowed to unambiguous guidance, helping the HIV cure enter clinical trials.

The recent response from the FDA was concise, and it presented a short list of specific items to implement and report; items that AGT is well-prepared to provide. The previous "fuzziness" in the review process was completely clarified, and the FDA provided focused feedback that greatly enhanced our discussions.
AGT now feels positioned to respond to the shortlist of items the FDA requested. We should be able to fully comply in a Complete Response to Hold (CRH) submission within the next 30 days. We can't say for sure what the FDA's next response will be, but we believe that we will provide the full set of data they requested and positively respond to the guidance in their May 20th review-committee letter. The FDA and AGT now seem aligned and in-sync regarding how to assure safety for patients and provide the greatest chance of a successful outcome to AGT's human trial of our HIV cell therapy cure!
HIV Cure Program Published In Molecular Therapy
Methods & Clinical Development
NIAID & AGT Peer-Reviewed Article
We have captured sufficient patent landscape (see four new patents below) necessary to begin publishing articles in peer-reviewed journals about our HIV cure program.
An article published May 3rd in Molecular Therapy is the most extensive, scientifically-detailed information we have ever released on our autologous cell therapy designed to cure HIV.
With our phase 1 clinical trial approaching, we hope that the release of this article will assist in educating and informing scientists, doctors, and the HIV+ community on the results of the years of work leading up to this point, and our confidence in an HIV cure. Articles like this one are an essential contribution to scientific research and discovery on HIV, and we are proud to do our part to contribute to the industry’s understanding of this insidious virus. https://www.cell.com/molecular-therapy-family/methods/fulltext/S2329-0501(20)30084-X#figures
How Covid-19 Has Impacted AGT & The Industry
The pandemic hasn’t affected the R&D progress of AGT’s programs, however, regulatory slowdowns that began earlier, have persisted and continued to lag for the gene and cell therapy industry. The addition of special programs and reviews at the FDA for Covid treatments and vaccines may have slightly exacerbated this issue. However, the gene and cell therapy industry is mostly a victim of its own success: becoming the hottest technology in pharmaceuticals and on Wall Street over these past few years. “Herd mentality” pervading institutional investors and investment banks has inspired (and funded) the creation of many new companies pursuing monogenic diseases utilizing a variety of gene editing approaches, and this has strained resources (especially FDA review) for everyone. The industry is flourishing, and the FDA has experienced the rapid surge of IND applications. We continue to interact and meet with teams from the FDA since they began working from home, and everything seems to be continuing to progress (and improve) on their end.
Testimony From Our HIV Scientific Advisory Board
Meet One of Our HIV Scientific Advisors
Dr. Hardy, A Johns Hopkins University Professor And HIV Expert, Shares His Thoughts On The Possibility For An HIV Cure Through Gene Therapy
Dr. David Hardy, a member of American Gene Technologies' (AGT) HIV Scientific Advisory Board, shares his experience and perspective on HIV cure research. In the past, he was constrained to treating the symptoms of the virus. Today, he is advising AGT as it pursues a gene therapy treatment intended to give HIV+ patients immunity to the disease.
In this video, Dr. Hardy shares that over 40 million people worldwide have been impacted by HIV/AIDS. He notes that HIV+ individuals are largely able to control the disease with the help of antiretroviral therapies, but the need for a cure remains great. He states: "I believe the final solution for a cure is going to involve gene therapy."

Phenylketonuria (PKU) Program Update
New Scientific Milestones
Poster Presentation
The PKU program recently reached some new critical scientific milestones which attracted potential partners into our data rooms. To learn more about our PKU program, see the poster presentation (click here) from our Director of Pre-Clinical Development Kelly Huang Ph.D. or read about it on our website (click here).
Licensing Interest In PKU
From Pharma Companies
Potential partners are currently reviewing our PKU program. A partnership in PKU would not only accelerate AGT‘s drug candidate into the clinic, but would also offer sustainable capital for all of AGT’s lead programs (including HIV and immuno-oncology). A large pharma partnership could provide external validation of our platform, creating momentum to build new collaborations with other large pharmaceutical companies. In the short-term, upfront cash from a partnering deal could merit the closing of our 2020 financing early with non-dilutive capital.
Patent Portfolio
Building A Picket Fence Strategy
4 New Patent Grants Brings AGT's Total Granted Patents to 12
In an emerging industry, creating a patent estate can be a critical to long term commercial value. We are working hard to ensure that we are well prepared for the coming years to stand firmly at the nexus of systems biology, genetics, and the tools and components required to modify the human genome, by capturing high-value patents for fundamental innovations in the use of viral vectors, methods for gene and cell therapy, discoveries, and molecules that have multiple applications across 1000’s of diseases.
Our picket fence strategy is designed to ensure multiple barriers of protection for our technology and drug development assets as the gene and cell therapy market rapidly evolves and as it becomes increasingly competitive. Over the last three years, you can see our team has been laying down critical patents in viral vectors, cell processing, infectious disease, and fundamental oncology discoveries. Click on the patent thumbnails below to take a deep dive into the four additional patent grants received so far in 2020.
We’re Moving To Upgraded, Larger Offices/Labs
Take a Tour of the Construction
Bigger Labs. Newer Equipment. Better Views
Blueprints to Our Future
The Facility
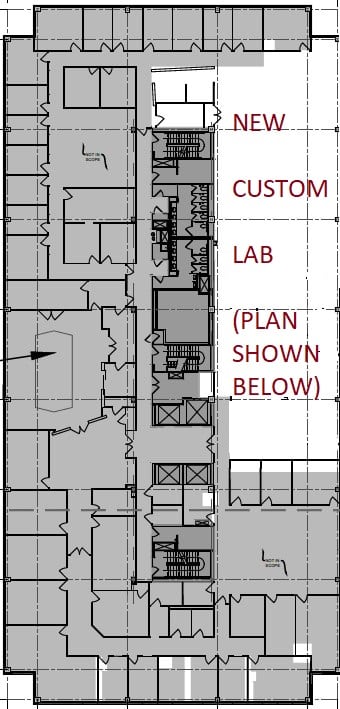
We are in the process of building out our “dream lab” and creative space for pursuing the AGT mission to reduce suffering and cure diseases with AGT’s extensive gene and cell therapy platform. We expect that our new offices should be ready in August.
This 27,000 square-foot site in Rockville will allow AGT to expand its team rapidly, efficiently, and purposefully as we hit critical growth milestones in the next 12 months.
To support the company’s expected future growth, the new building provides 50% more lab space, fully customized laboratory configurations, and advanced instruments to enhance our internal capabilities.
The lab is just over 6,000 square feet and includes a large, open footprint lab and Translational labs with 120 feet of windows permitting natural light and views over DNA Valley. Opposite the windows, a long 100% whiteboard wall stands ready for brainstorming and collaboration. There are nine enclosed labs, including five tissue culture labs. These are just some of the progressive, supportive resources AGT’s new facility will feature.
The laboratories were designed with the thoughtful input of our Chief Science Officer C. David Pauza, PhD to encourage a working environment that can maintain our scientific creativity while responding to changes that are critical for nimble growth in a rapidly evolving and expanding industry.
AGT’s new address, 9713 Key West Ave., Suite 500, Rockville, MD 20850, puts us at the center of the booming life science research arena. Considered the heart of DNA Valley, this area is rich with opportunities for biotech growth due to its proximity to the FDA, NIH, NCI, NIAID, local partnership opportunities, and one of the country’s most dense populations of PhDs and STEM-educated workers.
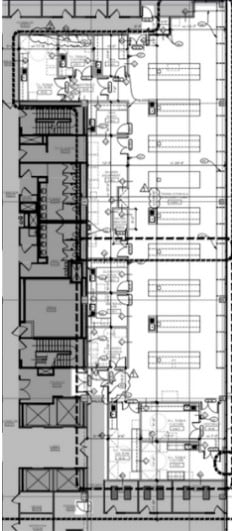
Across the street from Johns Hopkins Shady Grove Campus, the National Cancer Institute, and within a few miles of AstraZeneca and GSK, AGT’s new office will keep us connected to the pulse of the rapidly expanding and evolving biotech industry. We hope you will stop by to see our new home if you find yourself nearby.
We’re Hiring - Accelerating Our Science
Recent Hires

AGT continues its growth and recently hired two new positions to accelerate R&D. A new Senior Scientist has been hired to work in Preclinical Product Development. This new scientist will focus on moving R&D ”discovery” programs into the clinic. The company also hired a Senior Accountant to expand our finance department.
Open Positions
AGT has open positions:
Clinical Study Manager - Gene and Cell Therapy
Director of Business Development
In-House Legal Counsel
Industry Performance
This Year’s Biotech IPO Activity
Despite the broad financial markets correcting and retrenching, biotech is moving ahead and executing IPOs.
Given behavior during the last market crash you might expect IPO’s would have ground to a halt. Not so in biotech. While the numbers of biotech IPO’s are down, many are still being executed and performing well!
The Last Market Crash Impact on Biotech
Get Ready for a Surprise
Read Senior Biotech Analyst John Vandermosten’s article to review Biotech market activity from the 2008 stock market crash until now. You may be surprised by how it eluded the forces that impacted other sectors. From the last market crash to the COVID-19 market correction the BTK significantly outperformed the S&P 500 Read.
“AGT's Gene and Cell Therapy Explained”
Video Series
Featuring Videos from AGT Executives

AGT recognizes the importance of utilizing evolving technologies and continuing to invest in a talented workforce. As a result, AGT created this video series to help the industry understand gene and cell therapy R&D and to attract new talent to the company.
View the series on YouTube and Subscribe.
Cell and Gene Therapy Approach
Jeff Galvin, CEO of AGT
Gene therapy technologies and development are accelerating at an incredibly impressive rate. AGT is at the forefront of this revolution which is fueling the current boom in the Biotech market.
Watch Here


Cell Therapy Manufacturing
C. David Pauza, Ph.D., CSO of AGT
This video explains the ins and outs of cell therapy. Dr. C. David Pauza, Chief Science Officer of American Gene Technologies, explains how one tiny dot on a cell, a virus, can kill a person, but this dot can be changed from the infectious root of disease into curative genetic medicine. Watch Here
Progression of a Gene Therapy Company
Jeff Galvin, CEO and Irene Tennant, VP of AGT
What is an ”AGT person”? AGT has a corporate culture that provides a place for smart, motivated, hardworking persons to pursue their passion to help others by developing innovative pharmaceuticals that ”swing for the fences” and provide new hope for millions of patients. Learn more in this video.


Using Gene Therapy to Cure Diseases
Irene Tennant, VP of AGT
Irene Tennant, the head of clinical development and regulatory affairs at AGT, speaks about her role in the development of gene and cell therapies to treat human diseases. She works to progress potential therapies into the clinic, and ultimately to the bedsides of patients in need. Watch Here
About Gene Therapy
Jeff Galvin, CEO of AGT
In this video, Jeff Galvin explains how American Gene Technologies (AGT) uses genetic modifications in the body to change how viral diseases interact with the immune system. Watch Here

Bioneex Interview
Interview with CEO Jeff Galvin
By Dr. Smbat Rafayelan

Bioneex, a Biopharmaceutical R&D licensing platform for compounds and co-development partnerships, created an interview series of innovative CEOs and executives to provide value and education to its customers and the biotechnology industry.
Founder Dr. Smbat Rafayelyan featured AGT CEO Jeff Galvin as part of its series. In this interview, Jeff Galvin goes into detail on AGT’s programs and progress. Watch it here.
BioSpace
The Future of the Gene and Cell Therapy Industry
Interview with Jeff Galvin, CEO of AGT
This April, BioSpace interviewed Jeff Galvin as a thought leader to share his perspective on the rapid growth of biopharma and how gene therapy is creating a new paradigm in drug development.
In this article, he shares a perspective on the industry: “in the same manner that the personal computing industry grew and developed business models that fit the way products were sourced, constructed and delivered, a business model for biopharma is taking shape.”
Jeff has been personally committed to engaging the public by sharing the power of gene and cell therapy and what it means for the future of healthcare: curing diseases vs. treating them. Industry publications like BioSpace are recognizing the impact of gene and cell therapy and how leaders like Jeff Galvin will help shape the future of medicine. Read the article here.
Three New Blogs
Featured Stories Include:
Market Crash Impact on Biotech, 12 Coronavirus Stocks, & the Benefits of FDA Orphan Drug Designation
AGT with the help of Senior Biotechnology Analyst John Vandermosten, AGT’s Director of BD Norman Rogers, and its VP of Clinical Development and Regulatory Affairs Irene Tennant have written three new blog articles. These articles are intended to provide valuable and relevant information to our community. Please read and let us know if you enjoyed these articles or if you’d like to learn more about other topics in our industry.
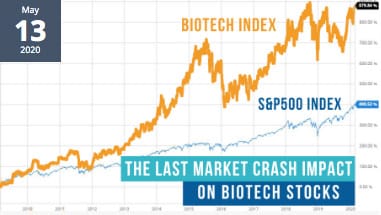
What Impact Did the Stock Market Crash Have on Biotech Stocks?
Gene Therapy Investing

Benefits of FDA Orphan Drug Designation: What You Need to Know
Rare Disease Research
12 Coronavirus Stocks To Watch As The Race For A Coronavirus Vaccine and Treatment Continues
Gene Therapy Investing
AGT Maintains Its R&D Progress During COVID-19
AGT Cares About Public Health and The Health of Its Employees
AGT cares deeply about the health and well-being of our community and our team. Many have been personally affected by the Coronavirus and we understand the gravity of this time in history.
AGT’s is headquartered in Maryland, where we are fortunate to have the strong, caring leadership of Governor Larry Hogan at the helm in these uncertain times. He and many other government officials and public servants throughout Maryland have risen to the challenge of the pandemic, tirelessly working to control the outbreak, preserve our economy, and plot a course back towards normal.


AGT’s is headquartered in Maryland, where we are fortunate to have the strong, caring leadership of Governor Larry Hogan at the helm in these uncertain times. He and many other government officials and public servants throughout Maryland have risen to the challenge of the pandemic, tirelessly working to control the outbreak, preserve our economy, and plot a course back towards normal.
We reached out to send a thank you to Governor Hogan that attracted many positive comments from Marylanders. We're confident of Maryland's future knowing that dedicated Maryland leaders continue to place public safety at the top of their priorities. Thank you to leaders, front-line workers, healthcare professionals, public servants, scientific experts, and all Marylanders who are working together to serve their neighbors and community during this immense global challenge.
AGT Remains at Full Speed During the Health Crisis
AGT is continuing full speed ahead on all its programs and has made a smooth transition to work-from-home, while implementing Maryland's and the CDC’s safety guidelines for essential workers in its labs.
AGT’s CEO had a background in software and internet, so the company had implemented digital collaboration software years ago. Existing systems made it seamless for the company to pivot to work-from-home when ordered to “shelter in place” by the State. AGT’s efficiency and productivity continue at pre-pandemic levels as our team collaborates remotely, holds meetings by video, and works in "the cloud".
Connect With AGT on Social Media
Interested in AGT News?
Please have a look at our latest press releases to see all of our progress and what we have been up to. You can also visit the newsletter section to read about all that American Gene Technologies has been working on.



