NEWSLETTER VOLUME 10

We’ve achieved a number of significant milestones this quarter, including the Food and Drug Administration (FDA) clearance to begin Phase 1 of our HIV cure program. The excitement hasn’t only been within our walls; AGT has appeared in publications and news stories across the country.
The AGT Team's commitment to our mission to help patients has inspired daily progress in all departments. The clinical team is coordinating with sites to enroll the first patient in the HIV cure trial in October. AGT's scientific team is processing nearly 100 patents that give us tools, components, and techniques to continue to address life-threatening diseases. Meanwhile, the business team at AGT continues to fund the company and build a commercialization path to bring our programs from the lab to patients.
The Phase 1 Trial of The HIV Cure Project
Clinical Update
The "REPAIR" (REstore Potent Antiviral Immune Responses) Human Trial
In August, AGT received an approval by the FDA to begin Phase 1, the first human clinical trial for AGT’s lead HIV program. The clinical affairs and regulatory teams have been preparing for this event for the past year. As soon as AGT received approval, the team initiated selected clinical trial sites to enroll the first participant in October.
Anisha Mannan, Director of Clinical Operations, provides an update on the trial status.
Enrollment will be conducted by Washington Health Institute, University of Maryland - Institute of Human Virology, and Georgetown University. The AGT103-T study details and recruitment status are published and will be updated to https://clinicaltrials.gov/. AGT anticipates the first infusion in this study to be performed by the end of this year.
This is a moment we have all been anticipating. We hope our efforts will help improve the quality of life of millions of HIV+ individuals, as well as advancing our capabilities in gene and cell therapy and other infectious diseases.
AGT103-T is a single-dose, lentiviral vector-based gene therapy developed to eliminate the need for lifelong antiretroviral treatment for HIV+ individuals and allow them to naturally suppress and eliminate viremia and latent, chronic infection. This is called a "functional cure" and should allow HIV+ individuals to live a normal life, without fear of progressing to AIDS, infecting others, or being reinfected with HIV, and to be permanently free of the toxic side effects of antiretroviral treatment. AGT’s Phase 1 trial will investigate the safety of AGT103-T, measure key biomarkers, and explore surrogate markers of efficacy. For more information, click here.
AGT In The News
Excitement About The Beginning Of Phase 1
The worldwide epidemic of HIV/AIDS has caused immeasurable suffering over the past four decades and has challenged the biomedical community with unique characteristics that make HIV particularly difficult to treat and harder to cure. Given the decades of struggle against the virus, stigma, and the daily strife endured by patients, AGT isn't the only one excited about beginning a human trial for a potential HIV cure. Here are a few examples of the 1000’s of stories, videos, tweets, and articles that resulted from our announcement of this important milestone.
ABC 7 News
ABC 7 News
Scientific Articles
Read And Share Some Of The Articles Highlighting AGT
AGT' HIV Functional Cure Project has recently been the subject of a peer-reviewed article in Molecular Therapy. The paper was co-authored by a team at NIAID who reproduced AGT's pre-clinical results in their lab, and the data is capturing the attention of HIV experts across the globe. Notable online science publications and influencers have also covered our research. Read and share some of these articles below:
Events
Watch and Share
AGT has held or participated in a number of virtual events this quarter to stay connected to the industry, HIV advocates, the media, investors, and other stakeholders powering this mission.
In AGT's virtual press conference, CEO Jeff Galvin and Chief Science Officer C. David Pauza, Ph.D. answered the media and public's questions regarding AGT103-T and its upcoming Phase 1 trial.
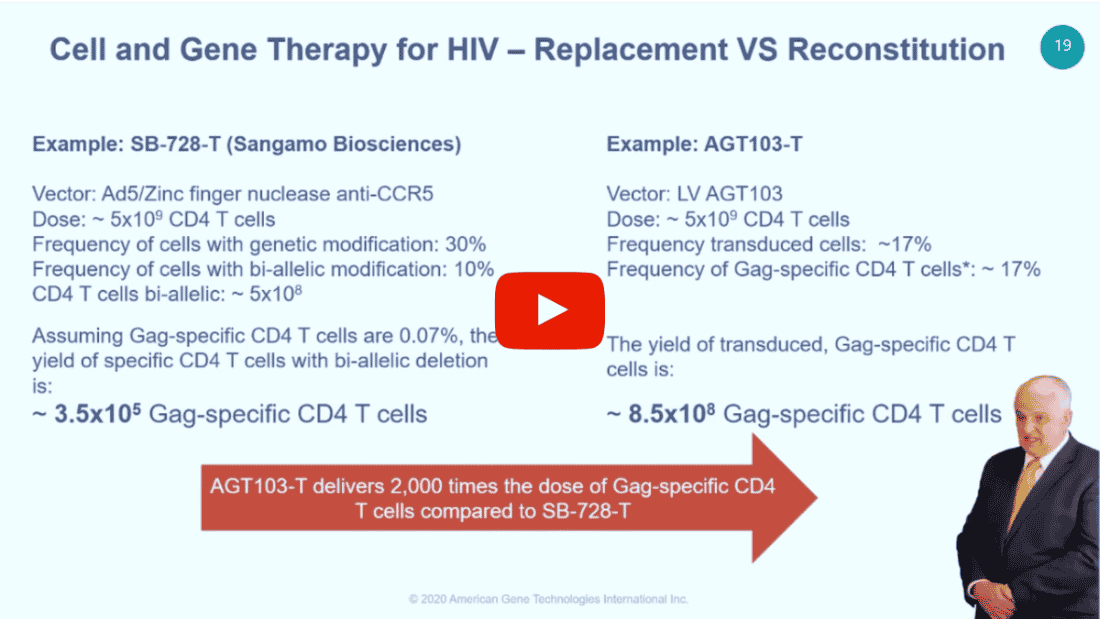
CEO Jeff Galvin has also been invited to present at a number of virtual conferences, including the Data Bridge Conference Series on Cell and Gene Therapy. Watch the recordings below.
Peer-Reviewed Article
See Our New Offices
Custom Lab Space
This Is Where Creativity Cures
AGT has settled into its new location across from the National Cancer Institute (NCI) campus and Johns Hopkins in the biotech corridor of Maryland. We're excited to share a walkthrough video from move-in day to our new offices. See our new location on Google Maps.
This 27,000 square-foot site in Rockville will allow AGT to expand its team rapidly as we hit critical growth milestones in the next 12 months.
Our custom-designed lab features a large, open footprint for R&D and Translational labs. Watch this video to view the new space or read our press release.


This new facility will support AGT's HIV Cure, PKU, and immuno-oncology lead programs, while providing expansion capacity for additional product and pipeline development.
At its core, AGT’s mission is to develop therapies that seek to reduce suffering and prevent premature death from serious human diseases. This new location will be the center of that mission. It contains all of the tools needed to continue our leadership in the industry and to develop our technology for the betterment of millions of lives.
Special Thank You
Reaching The HIV Cure Milestone
Message From The CEO

Thank you to everyone reading our newsletters and powering our mission. Whether you are retweeting our news, working alongside us, or have made an investment in AGT; I am grateful to you. It is an incredible honor to be part of this revolution in medicine.
I was excited by the potential of gene therapy when I was first introduced to this technology over a decade ago.
Now I am fortunate to be involved in the future of medicine alongside you and among the many companies that are pursuing cutting-edge technologies in gene and cell therapy that represent new hope for so many people in need.
Thank you for coming this far with us. I think we are getting close to demonstrating AGT's unique capabilities and our potential to efficiently create programs and products with significant clinical results and important benefits for patients.
Becoming a clinical-stage company is a huge milestone for AGT. We are one step closer to a possible cure for HIV and each step we have made, over our 12-year history, has brought us more tools and more capabilities to craft potential solutions to a wide variety of infectious diseases, monogenic disorders, and cancers. I have marveled at previous successes in gene and cell therapy such as Luxturna, Zolgensma, Kymriah, Yescarta, and others that are already delivering effective, highly valuable treatments to patients. With success in the HIV program, gene therapy will cure not just another rare disease, but a disease that affects over One Million Americans.
Jeff Galvin, CEO
American Gene Technologies
To learn more about the portfolio and pipeline at American Gene Technologies, visit our website at www.americangene.com