NEWSLETTER VOLUME 8

AGT’s HIV cure program is nearing a major milestone: Investigational New Drug (IND) submission. The IND is the key document that reports the safety data the FDA requires prior to a human trial. If the document meets the expected criteria, AGT will be authorized to begin the phase I human trial. At this time, the IND is on track for submission, and pending acceptance from the FDA, the human trial is expected to start during Q4 2019!
AGT has made other important accomplishments. It signed a Research Collaboration Agreement with the National Institute of Allergy and Infectious Diseases (NIAID) whose researchers will study the mechanism of action of AGT's proprietary, autologous cell product AGT103-T to further understand and characterize its potential impacts on HIV (press release).
Early this year, AGT completed the transfer of the cell process manufacturing from Mitenyi to Hitachi Chemical Advanced Therapeutics Solutions (HCATS). HCATS was successful in repeating the final (locked) cell protocol and in obtaining the same results within the developmental lab, allowing the project to resume at full speed. Over the last few months, six additional validation and training runs were completed and AGT is currently moving to the GMP suite for the final run necessary to complete the IND!
While AGT advances its science, it continues to protect its research discoveries. It recently gained two new patents: one protecting its HIV cure asset (press release), and the other protecting its method to cure several types of cancer (press release). In only 17 months, the company has been granted a total of 7 USPTO patents. It has several additional patents pending, and more than 20 patent families represented by filings with the USPTO and international patent offices.
To further accelerate and strengthen the ImmunoTox program, AGT added five new, internationally recognized scientific advisors to its team and formed the Oncology Science Advisory Group (OSAG). The new members are from three different nations and are all leading researchers in their fields.
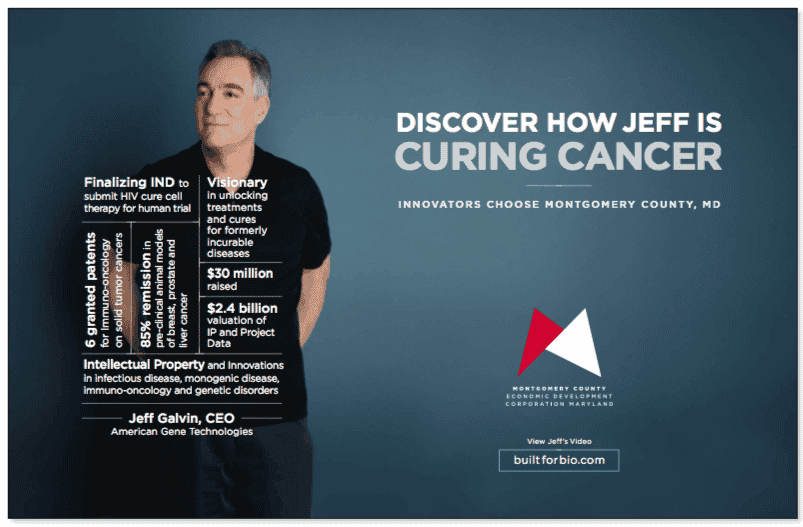
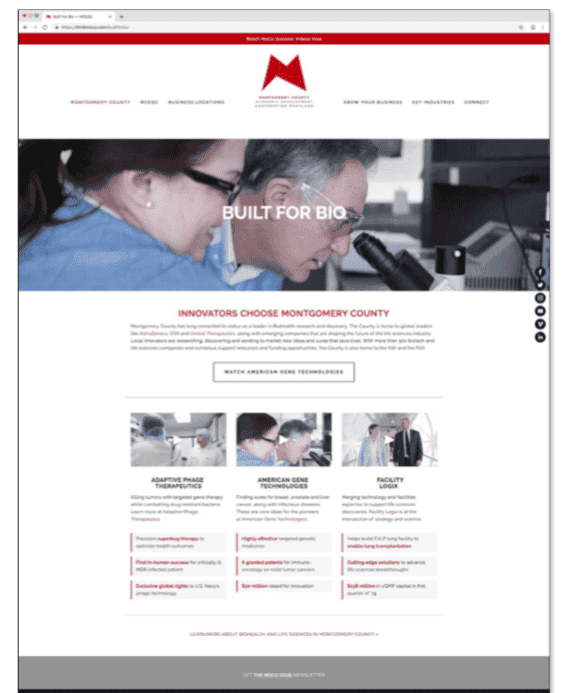
These researchers weren’t the only ones who recognized AGT’s advances to develop life-changing therapies; the Montgomery County Economic Development Corporation (MCEDC) chose AGT and CEO Jeff Galvin to be one of three CEOs to participate in its “Built for Bio:” a major marketing campaign to attract C-level talent to Montgomery County, Maryland. Watch MCEDC’s video on AGT:

HIV Progress
Two key scientists in the HIV Cure program spoke about their work and confidence in AGT’s HIV program. AGT’s Chief Science Officer, Dr. David Pauza, has a 35-year career in researching and treating HIV. He is determined to find a cure for the disease. AGT’s Scientific Advisor Dr. Ely Benaim is deeply familiar with AGT's work and believes the cure may be near. Dr. Benaim was formally the CMO on an important HIV study at Sangamo Therapeutics. His interest and optimism over AGT’s HIV program is appreciated by the whole team. Watch these videos to hear their personal thoughts:
C. David Pauza, PhD
Chief Science Officer, AGT
“We have this golden opportunity now, here at the real beginning of the age of genetic medicine. We have the opportunity to participate and be pioneers in really curing things.”
Ely Benaim, PhD
Scientific Advisor, AGT
Chief Medical Officer of NovoCure
“AGT brings a new form of therapy really trying to push into curing HIV...eventually if they are successful, it will mean the cure of HIV...”

AGT is getting close to its anticipated clinical trial for HIV. The current forecast ahead is to submit the IND and begin clinical trials in Q4 2019, provided the IND is approved by the FDA. If AGT experiences schedule delays, it anticipates a difference of weeks versus months because the company has so little left to do to finalize its IND. The AGT team is confident it will achieve its upcoming milestones. This is a very exciting time for AGT and all of us!
Furthermore, the company’s two USPTO patents help ensure the protection of its unique technology and cell process intended to cure HIV.
In AGT’s Research Collaboration Agreement with NIH: National Institute of Allergy and Infectious Diseases (NIAID), AGT will provide genetically modified T-cells created during the development of its proprietary cell product for clinical trials. These cell products are highly enriched for HIV-specific CD4+ T cells, and are modified by AGT’s proprietary lentivirus vector, to protect cells from HIV-mediated depletion (in other words, HIV T cells that are immune to HIV and can’t be killed by the virus). The collaborative studies will help to characterize these enriched cell products and define their antiviral effects. NIAID has reproduced AGT’s data, thus further validating AGT’s technology for creating a natural protection against HIV.
AGT recently recruited the Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM) as the new clinical site for its non-IND study, AGT-CS168, to collect clinical blood specimens from HIV positive individuals. AGT is using these specimens to: (1) continue to validate the patient-specific AGT103-T therapeutic manufacturing process and (2) complete the final cell run under good manufacturing practices (GMP), to generate the final data needed for its Investigational New Drug (IND) application (read press release).
AGT is on track to submit its IND application to the FDA within months! Transferring its cell manufacturing process and final, FDA required pilot runs to Hitachi Chemical Advanced Therapeutics (HCAT) has enabled AGT to accelerate its timeline to submission.
AGT completed the first stage of technology transfer with HCAT in January when it repeated and re-validated the locked cell protocol and obtained the same results as formally reported by the previous cell processor. As a result, AGT is more confident than ever in its cell product. AGT is also more confident in HCAT’s ability to supply the required FDA compliant data.
In April, AGT moved into its second phase of work with HCAT, training for a clinical trial in a Good Manufacturing Practice (GMP) suite. This training stage continued for two months. During this time, AGT both drafted all documents needed for GMP manufacturing and manufactured three additional cell product lots for its expected trial. AGT anticipates one more cell product manufacturing run with full GMP paperwork before submitting the IND. AGT is on track to complete the GMP trial run for the FDA, submit its IND application, and begin human trials by the end of this year.
In an effort to serve the HIV/AIDS community, AGT provided sponsorship funds to One Tent Health, an organization that provides 15 minute HIV testing for individuals at high risk of getting the virus in Washington, DC. Even with a cure for HIV it will be critical to expand testing and to locate everyone who needs it. AGT is thinking ahead to the day where HIV may be eliminated, in our lifetime.
There are other things going on in HIV research that continue to extend hope for that day HIV will no longer be a threat to mankind. This March, for the second time in history, an HIV infected person was cured of the virus. This patient was cured after receiving a bone marrow transplant from a CCR5-Δ32 donor, who has non-functional copies of the CCR-5 gene. A functional copy of this gene produces a handle by which HIV can enter cells. Without this handle, missing from the CCR5-Δ32 donor, HIV cannot enter cells and the immune system attacks HIV as it would a flu or cold virus. AGT uses this same concept to develop its cell therapy designed to cure HIV (press release). Bone marrow transplant are frequently deadly and can never become a broadly applied cure strategy. However, the development of AGT’s cell therapy is expected to provide the same therapeutic benefit without the need for a costly, traumatic need for a bone marrow transplant.
Cancer Cure Progress
AGT began the quarter on a high note when it was granted its fifth immuno-oncology patent (10,137,144) by the USPTO. This is another critical win in protecting AGT’s unique methods to eliminate solid tumors (read more about AGT’s cancer program). If you aren’t familiar with how AGT’s therapy works, watch this 5 minute video of CEO Jeff Galvin explaining it (click here).
AGT’s Oncology Science Advisory Group (OSAG) boasts five new, renowned members of the scientific research community: Dr. Miroslav Malkovsky, Dr. Jean Jacques Fournié, Dr. Marc Bonneville, Dr. Julie Déchanet-Merville, and Dr. Yoshimasa Tanaka (click here to read their bios). The new members will assist AGT in testing our gamma delta T cell cancer therapies to validate its effectiveness in animals and humans.
AGT’s ImmunoTox vector is progressing well towards a human proof of concept study in liver cancer (Hepatocellular carcinoma (HCC)). AGT expects to expand ImmunoTox’s application to several other solid tumors after demonstrating this proof of concept in HCC. ImmunoTox’s clearance of secondary tumors and metastases when treating HCC in mouse models is exciting and may be the key to unlocking the next generation of cancer care. AGT is also proud to have our long term advisor Dr. Dean Felsher, MD, PhD, as both a clinical and scientific advisor. Dr. Felsher is Dean of Translational Medicine at the Stanford Medical School, which we anticipate will be a trial site in late 2020 or early 2021.
Over the last six months, AGT increased its collaboration with scientific advisor Robert Clarke, PhD Professor of the Department of Oncology and Co-Director of the Breast Cancer Program at Georgetown University Medical Center. This collaboration is intended to gather data on AGT’s immuno-therapy efficacy in breast cancer. ImmunoTox has shown high potency in mouse models of human cancers. Dr. Clarke is simulating typical human breast cancer conditions in mice to further study ImmunoTox’s effectiveness in breast cancer.
Learn More About How AGT’s Cancer Therapy Works:
- Jeff Galvin, CEO at American Gene Technologies, explains AGT’s method of fighting cancer: Watch Video.
- CEO Jeff Galvin explains the power behind AGT’s cancer research and its groundbreaking discoveries: Watch Video.
- CSO David Pauza “Gamma Delta T Cell Therapy for Cancer: It Is Good to be Local:” Read Article.

PKU Cure Progress
AGT is currently developing a cure for phenylketonuria (PKU), a debilitating inherited disease affecting 1 in every 13,500 children born in the United States. Jeff Galvin, CEO and Founder of AGT, spoke at the Orphan Drug World Congress USA in Washington, D.C. April 11th about AGT’s preclinical progress on a potential PKU cure. AGT’s PKU cure program was recently granted an Orphan Drug Designation by the FDA (press release). AGT’s technology demonstrates the potential to address an unmet medical need in treating this rare disease.
AGT is in discussion with potential strategic partners for its PKU program. Current data for this therapy is extremely promising, and AGT is expecting to release additional data this summer.
The Orphan Drug Designation, offers benefits to AGT, including: tax credits of 50% of the clinical drug testing cost awarded, eligibility for market exclusivity for 7 years, and waiver of NDA/BLA application fee (something that can have a value of 2.2 million dollars). The Orphan Drug Designation allows AGT to move forward even more efficiently to develop this important treatment.

Two New USPTO Patents
AGT continues to build an impressive patent portfolio. Click on the below links to read the patents and press releases.

Patent No. 10,233,464
“Methods and Composition for the Activation of Gamma Delta T cells”

Patent No. 10,137,144
“Methods and Composition for the Activation of Gamma Delta T cells”
Press Release

Press
This quarter, AGT received press coverage for its notable science and efforts to find gene and cell therapy cures for HIV, cancer and PKU. Appearing in multiple different media outlets, AGT is growing its brand.
- C&EN Magazine Volume 97, Issue 27 mentioned AGT in their published article “How Phenylketonuria, A Once-Neglected Disease, Became A Proving Ground For New Drugs”
- WFED 1500AM Federal News Network’s Executive Leadership Radio Show Interviews Jeff Galvin (click here to listen)
- C&EN Magazine Volume 97, Issue 19 mentioned AGT in their published article “With several long-acting HIV treatments in the works, the newest drugs emphasize convenience”
- Wonk FM, an iHeart Radio channel, interviewed Jeff Galvin (click here to listen)
- Site Selection Magazine published an article on AGT and Jeff Galvin: “The Future of Health Care Is Here” (click here)
News Releases
- HIV Cure Program Patent Granted To American Gene Technologies (press release)
- AGT Launches Oncology Science Advisory Group to Accelerate Research For A Cancer Cure (press release)
- AGT Granted Fifth Patent Protecting Its Immunotherapy Cancer Cure Asset (press release)
- Second Patient Cured of HIV and The Implications for American Gene Technologies’s AGT103-T (press release)
- American Gene Technologies Signs Research Agreement with NIAID for Experimental HIV/AIDS Cure Strategy (press release)
- American Gene Technologies To Attend 2019 J.P. Morgan Healthcare Conference (press release)
- Built For Bio: Jeff Galvin, CEO & Founder of American Gene Technologies - Jeff Galvin was featured in the Built For Bio advertising campaign

AGT Selected By Montgomery County, MD
American Gene Technologies and Jeff Galvin were featured in Montgomery County Economic Development Corporation’s Built For Bio advertising campaign. This campaign has appeared or is scheduled to appear in: Philadelphia International Airport, Logan International Airport, Washington Business Journal, Biotech Daily Magazine, MCEDC website, and more. To see the original art and content used in the campaign see the below video and graphics:

Highlight Events Attended By AGT
Jul 24
Learning Undefeated YESP Camp Visits AGT
At American Gene Technologies

Jul 7
WFED 1500AM Federal News Network Executive Leadership Radio Show Interviews Jeff Galvin

May 17
Washington, DC’s WONK FM & iHeart Radio Interview Jeff Galvin on Show: Emerging Technologies

May 8-10
Jeff Galvin Attended
J.P. Morgan CEO Retreat
Biscayne, Florida


Apr 10-12
Jeff Galvin Speaks at World Orphan Drug Congress USA 2019
Gaylord National Harbor Hotel, Oxon Hill, MD

Apr 8-9
BioHealth Capital Region Forum 2019
Medimmune - AstraZeneca, Gaithersburg, MD

Mar 21
Jeff Galvin Speaks at Alliance for Regenerative Medicine Investor Day
Metropolitan Club, New York, NY

Mar 21
Hottest Technologies & Innovations of Montgomery County
At American Gene Technologies with co-hosts Leadership Montgomery


Feb 5
JP Morgan Visits AGT
at American Gene Technologies

Jan 31
AGT Meets Maryland Senator & MTC’s Leadership Dinner and Annapolis Day
Annapolis, Maryland

AGT aligns with Maryland legislators who support biotech growth in the State.

Jan 15
Jeff Galvin Speaks on Maryland’s Vaccine & Immuno-Oncology Panel
Glaxo Smith Kline, Rockville, MD


Jan 8
Redefining Early Stage Investments (RESI)
San Francisco, CA

Upcoming Events
Sep 9-10
Janney Montgomery Scott Healthcare Conference
The Union League Club, New York, NY

Sep 11
Jeff Galvin Speaks to College of Natural and Mathematical Sciences UMBC
Rockville, MD



Nov 7
Biomedical Science & Engineering (BSE) Grand Opening & STEM Expo
UMBC at Universities at Shady Grove, Rockville, MD


Industry News
Quarterly Gene & Cell Therapy Breakthroughs
- Iovance Therapeutics jumps 36% in stock as their TIL drug out performed Merck's, Keytruda drug for cervical cancer. (The Motley Fool)
- Experimental Gene and Cell Therapy Finds Solid Ground with $150 Million Raised by UBS Oncology Impact Fund and F2 Ventures. (Xconomy)
-
While startup companies tend to struggle under the pressure of costly manufacturing, ElevateBio is investing in the future of these startups. Startup gene and cell therapy companies will not only find the needed space to grow but have the funding to further explore curable options.
-
- Thermo Fisher Aims to Buy Brammer Bio for $1.7 Billion to Strengthen Their Research Into Gene Therapy. (Zacks)
- Johnson & Johnson is Gearing Towards the Prevention of Eye Diseases By Buying Out MeiraGTx. Leading J&J to top $50 Billion Stated by the RBC Capital Markets. (Investor’s Business Daily)
- Signet Healthcare Partners Invests in Vigene Biosciences, Inc to Create New Facility To Meet Demands With 51,000 sq. ft. laboratory and suites. (Cision PR Newswire)
-
The new additions will include a 51,000 sq. ft. laboratory, 5 cGMP manufacturing suites, thus leading to 10 production facility suites all 30,000 sq. ft.
-
- Kite Pharma Expands Their Manufacturing With A New 20-acre Site Plan in Maryland. (MedCity News)
-
Kite Pharma continues to focus on getting their product to patients without wasting time.
-
- Vibalogics makes investment in viral manufacturing to meet increasing demand. (BioPharma - Reporter)
-
Vibalogics increases its single-use bioreactor and purification capacity with a new manufacturing line to meet demand for oncolytic virus and viral vector manufacturing services.
-
- Vericel Corporation, JCR Pharmaceuticals Co. Ltd, MEDIPOST, and Osiris Therapeutics, Inc. are Part of the Growing Global Cell Therapy to Reach $7.9 BN By 2025. The Steady Growth Increase is Showing a 5.34% for its Predicted Forecast Period. (GlobeNewswire)
- Investors are Seeing an Upsurge with Mustang Bio of Up to 10.29% as Hedge Funds Buy More of the New York Company Stock. (Mayfield Recorder)
-
Mustang Bio Inc, based in New York is working on to commercialize the use of immunotherapy products by helping a patient’s own immune system to fight back cancerous cells.
-
- If CRISPR Gene Editing Finds Cure for Only 10% of Their Patients, the Staggering Annual Sale is $27 Billion and Growing. (Investor Place)
- The Cell Therapy Market Bridges the Gap with Big Pharma/Bio-Pharma to Expand to $8.21 Billion by 2025. (R&D Mag)

Social Media
Follow Us!
Follow AGT on social media to track the day to day progress the company makes in its ultimate goal of transforming lives with genetic medicines that will rid the body of disease. Join AGT’s social community to be a part of the fight to cure HIV, cancer, and PKU.
Please click and follow AGT on its social media platforms:
Visit key pages mentioned in this newsletter: