Zacks SCR: “American Gene Technologies: The Software Revolution For The Next 100 Years”

Published by: Zacks Small Cap Research (SCR) | Written by: John Vandermosten, CFA
Download The Full PDF
American Gene Technologies (AGT) is a genetic medicines company developing a portfolio of therapies that employ the company’s proprietary lentiviral vector (LV) platform to focus on disease in areas of unmet medical need.
AGT’s pipeline consists of therapies for three different indications. The most advanced is cell and gene therapy to cure Human Immunodeficiency Virus (HIV), designated AGT103-T, which employs an ex vivo cell modification process capable of altering HIV-specific CD4 cells, which are then returned to the patient’s body to clear the infection. Following a pre-IND meeting with the FDA in October of 2016 and additional lab and IND preparation work, AGT expects to submit an IND in 2019, followed shortly after by entry into the clinic with its HIV candidate.
The second program is an in vivo gene therapy to correct the inherited metabolic defect phenylketonuria (PKU). This candidate uses a lentiviral vector to make a permanent change in a patients’ liver to repair the defective phenylalanine hydroxylase (PAH) gene. In addition to fixing the defective gene, the therapy also suppresses expression of mutant genes which are generating misfolded proteins that can interfere with the new sequence. The program was granted an orphan drug designation from the FDA.
The third program is an immuno-oncology program for the treatment of solid tumors. The therapy, based on the ImmunoTox platform, activates the target tumor to produce a stimulatory phosphoantigen. This in turn will activate the immuno-surveillance by γδ T cells and increase their cytotoxic activity.
AGT is platform-focused and frames its approach as writing software (DNA) onto the body’s operating system (cellular machinery). These efforts are supported by an extensive intellectual property protection portfolio extending to 2039. Corporate strategy employs a modular approach where the company’s portfolio of vectors and genetic cargo can be tailored towards specific applications, dramatically reducing the time and cost of new therapies while maintaining a high level of safety.
American Gene Technologies
Technological innovations advance science for the benefit of society and also drive the development of new drug therapies and treatments. In the last several decades, the human genome has been decoded, computing power increased over 10 million times1 and we have gained the ability to modify cellular machinery to express proteins essential for life and health. There have been abundant developments in the life sciences including advances in gene editing, approvals in chimeric antigen receptor T (CAR-T) cell therapy and interference RNA among many others. Standing with this impressive group is gene and cellular therapy, which may address many of the shortcomings present in its distinguished therapeutic peers and also cure disease rather than serve as a chronic treatment.
After a long, intense and lucrative career in the software and computer industry and a few years taking a well-deserved break, soon to be CEO Jeff Galvin decided to direct his attention towards finding interesting projects that could benefit from his guidance. Jeff desired a more relaxed role advising entrepreneurs on business plans and serving as mentor. He knew his experience in the information technology space would help him identify innovative companies and he let the entrepreneurial community know he was looking. He felt that the lessons learned in the Internet, software and computer industry could be adapted to help develop something truly consequential and on the forefront of technology. In 2006, Jeff met with numerous entrepreneurs to explore possibilities and projects ranging from developing new mobile applications in China to building DNA arrays that could function as diagnostic tools, to developing an aluminum alloy to store hydrogen. However, none of the opportunities that Jeff came across had all of the elements he sought: significance, scalability, efficiency and an advantageous entry point in an area where his expertise could be a competitive advantage.
Then, he received a proposal from a National Institute of Health lab that was closing due to changes in regulations. It was developing viral vectors and plasmids as a way to cure lysosomal storage diseases. After receiving an invitation to take a look, Jeff immediately hopped on a plane and departed California so quickly that he only had a polo shirt and slacks when he arrived on that auspicious snowy day in Bethesda, Maryland. The ideas presented at his first meeting delved deeply into viral vectors and how they could be used to repair absent or defective genes, essentially rewriting faulty DNA. While absorbing this information, Jeff understood the possibilities in terms of his information technology background where a data medium (viral vectors) could be used to deliver software upgrades (new genetic code) to the human operating system (cells). Instead of 01100001, it was AGTC. Although he did not know it at the time, the lab director was a distinguished biochemist who had created effective therapies for Gaucher Disease and Fabry Disease leading to the Lasker Award in 1982. This scientist’s work had led to the development of an enzyme replacement therapy later commercialized by Genzyme which was worth billions. His name was Dr. Roscoe Brady and at this point in his career he was retiring from the National Institute of Health (NIH), closing his lab and searching for someone interested in advancing the lentiviruses and plasmids that the lab had developed.
As Dr. Brady explained his work and shared the capability of lentiviral vectors plus a library of advanced plasmids he had created, Jeff immediately had a vision for the future of medicine. Seeing it through the lens of a software executive, lentiviral vectors were the media on which DNA software could be delivered. The human cell contained an operating system that could be debugged and reprogrammed to fix errors that prevented production of necessary proteins for human health. Jeff saw the next 100 years of the software revolution written in the DNA of our cells.
After a period of negotiations which yielded conditions favorable to robust company development, the viral vector program and all of its supporting assets were transferred to the new entity and American Gene Technologies (AGT) was born. For the next seven years, the company focused on conducting the bench work necessary to prove the platform and position the company for the future of gene and cell therapy in order to disrupt the trillion-dollar pharmaceutical market.
In AGT’s early years, interest in gene therapy from the large pharmaceutical companies and institutional investors was low. Early mistakes and failures had resulted in high-profile accidents that cast a shadow over the industry and oriented many biotechnology companies towards more established drug-development approaches. Along with Jeff’s personal financing, AGT was able to compete for NIH’s small business innovative research (SBIR) grants during this period and the company conducted proofs of principle in 15 different disease areas. Jeff’s experience had established that it was critical to validate the technology as thoroughly as possible in the lab, before taking it to the clinic. As the work moved deeper into the preclinical stage, the strategy began to yield drug candidates demonstrating a predictable clinical path, a high degree of efficacy in the clinic, and exposure to large end markets. This analysis yielded the three indications where AGT is now developing its candidates: HIV, PKU and cancer.
Tools of the Trade
Many diseases can be traced back to a missing, defective or unneeded protein coded by our genes. Sometimes these errors can be addressed by changing the environment to impact their expression and in other circumstances small molecule medicines or biologics can be applied to compensate for the problems they cause. However, none of these approaches addresses the core of the issue and permanently resolves the condition or represents a cure for a damaged or defective gene.
The first successful in-human gene therapy patient was a young girl treated in 1990 for adenosine deaminase (ADA) deficiency, a genetic disorder that can cause severe immunodeficiency and leaves the individual without immune protection. The four year old received the ex vivo gene therapy in a multi-step process that extracted autologous white blood cells, modified them in the lab with retrovirus containing the correct gene and then returned the cells to the patient. The procedure successfully addressed the disease and the young girl went on to live a productive life. Despite early victories, gene therapy has also faced some difficulties. There were several incidences of gene therapy patients developing leukemia due to oncogenic effects and Jesse Gelsinger, who suffered from ornithine transcarbamylase deficiency, died in a trial managed by the University of Pennsylvania. This led to a review of many other gene therapy trials underway leaving a cloud of suspicion over the technology for several years after the events. However, research continued to progress and in 2012 gene therapy Glybera was approved for a form of pancreatitis and in 2016 ex vivo stem cell gene therapy Strimvelis was approved for ADA-SCID patients. While both of these drugs broke new ground from a scientific perspective, their cost was so high that they were initially commercial failures. Other approvals include two CAR-T therapies in 2017 for blood cancers (Kymriah and Yescarta) and Luxturna for a form of blindness in 2018 which is the first in vivo gene therapy approved in the United States. These efforts have broken new ground and familiarized the scientific community and the regulatory authorities with these exciting approaches, helping set standards for safety and protocols for approval.
Gene Therapy – Evolution and Parameters
Gene-based therapy relies on designing a delivery system that introduces a functional copy to replace a mutated gene in a tissue or organ of interest without causing harmful side effects.
Exhibit II - Synthetic Lentivirus as a Viral Vector2
Click image to view larger
Gene therapy is the delivery of DNA to the genome of a patient’s cells as a treatment for disease. The transfer of desired genes into the genome does not spontaneously occur. A delivery mechanism is required and this is performed with a vector, which takes the form of a virus, bacterium or plasmid. The selection of the proper vector exploits naturally occurring agents that have evolved to deliver a segment of DNA to a cell.
There are two types of gene therapy, somatic and germline. Somatic gene therapy transfers the desired genetic sequence into somatic cells, avoiding integration into any cells that can be inherited by offspring. Some genetic disorders being investigated in this category include immunodeficiencies, hemophilia and cystic fibrosis. Germline gene therapy modifies a section of DNA that produces sperm or eggs and can be passed on to a patient’s children and future generations.
Challenges Associated with Gene Therapy
- Ethical issues: Regulatory authorities do not allow germline gene therapy. While it could benefit future generations from exhibiting the disease, it might have long-term negative implications that are not yet understood.
- Minimum effective dose (MED): It is important to determine the minimal pharmacologically effective dose for therapy that will mediate targeted delivery of the functional gene.
- Long-term implications: Safety remains the overarching concern for gene therapy. Gene therapy is in its nascent stage and its long-term implications remain unknown.
- High cost of therapy: Pricing the treatment based on whether it is a therapy or cure has remained a challenge. Nevertheless, treatment costs run high and compensation has been designed based on outcomes rather than volume.
Virus as a Vehicle for Gene Therapy
Since the 1970s, researchers have been investigating methods to engineer a carrier (vector) to deliver a novel gene to a target cell. Due to their ability to efficiently infect cells, viral vectors have been favored for transduction and to deliver a desired DNA sequence. Another approach for delivery is to encapsulate the DNA in a fatty liposome that has the capability to move across cell membranes to release the DNA. However, using liposomes as a delivery mechanism is less efficient than using a virus, as the encapsulated DNA does not target a specific cell. Naked DNA delivery and polymer-based delivery are yet other approaches that have been used.
The first target for gene therapy was in the area of inherited single-gene defects. During the early days, there was much the scientific community did not know. The use of some vectors, especially those that are immunogenic, have resulted in fatal and other severe consequences. In September 1999, 18-year old Jesse Gelsinger, who suffered from a partial deficiency of ornithine transcarbamylase was administered an adenovirus vector. Shortly after the treatment was given, he developed a fever and exhibited symptoms of liver injury. He later died of multiple organ dysfunction syndrome resulting from the excessive inflammatory reaction to the adenovirus vector. A short time later, children with X-linked severe combined immunodeficiency were infused with modified autologous hematopoietic stem cells that had been transduced with a murine leukemia virus (MLV) vector. Some of the patients treated with the vector subsequently were diagnosed with a leukemia-like disorder due to integration of the retroviral vector near a proto-oncogene locus. Subsequent reviews of these severe and negative side effects led to improvements that have enabled subsequent gene therapy programs to avoid these problems.
Much has been learned since the early days and viral vectors have emerged as safe and effective delivery vehicles for gene therapy. Some of the hurdles that have been surmounted include low immunogenicity and immune response, low genotoxicity, highly efficient and accurate delivery and stable genetic correction of target cells. Advances have also prevented the propagation of viruses through a number of methods including self-inactivating (SIN) vectors where the long terminal repeat (LTR) is modified.
Types of Viral Vectors
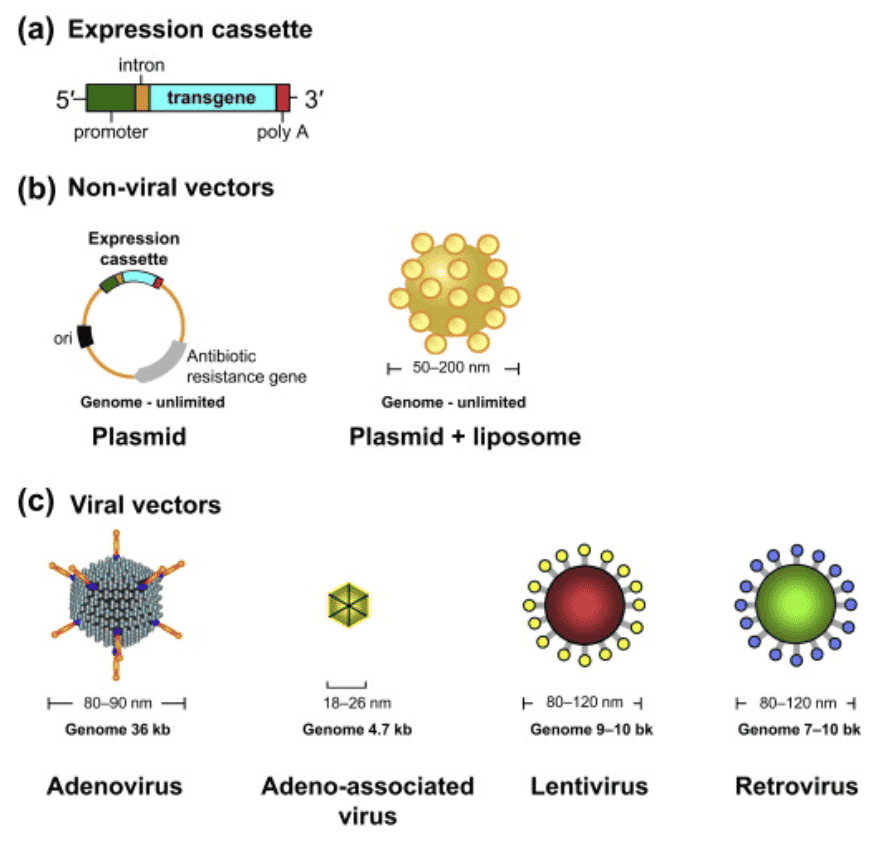
The application of viral vectors is disease and condition specific. The distinction is dependent on whether the viral genome integrates into the host’s chromosome (retroviruses and lentiviruses) or remains in the extrachromosomal region (adenovirus, adeno-associated virus, herpes virus). Integrating viruses are most widely used in therapies due to their ability to persistently express a therapeutic transgene. Although there are many types of vectors that are used for gene therapy, a few have distinguished themselves as leaders in the space which we detail below.
Integrating Virus
Retrovirus: A retrovirus produces a DNA version of its genome upon entry into cells, which is inserted directly into the chromosome of the host cell. The most widely used are the retroviral vectors based on Murine Leukosis virus, which were the first to be developed.
Lentiviral vectors (LV): Lentivirus, a subtype of retrovirus, can naturally penetrate an intact nuclear membrane and is capable of gene transfer to both dividing and non-dividing cells. Lentiviral vectors are primarily used for ex vivo hematopoietic gene delivery, where patient cells are removed and transduced with the viral vector, resulting in modified cells that can be transplanted back to the patient. In many cases a SIN LV will be used to reduce errors as it removes the promoter and enhancer thus reducing the impact on adjacent genes. In vivo (direct) lentivirus vector delivery is entering clinical studies especially for gene modification of the liver.
Non-Integrating Virus
Adeno-associated virus: The genome of the non-integrating virus (AAV) does not undergo site-specific integration in the host DNA but is transiently present as an extrachromosomal element in the nucleus of transduced cells. AAV strengths include its ability to infect both dividing and non-dividing cells and mild immune response from exposure. The number of people treated with AAV is small in comparison to those treated with T cells modified ex vivo with lentivirus. A downside to AAV is that it is not possible to insert large genes into an AAV as its cargo carrying capacity is limited to 4.5 kilobases and human safety data is still limited.
Herpes simplex-1 virus: Since HSV is an infectious virus, it is a naturally efficient vehicle for the delivery of genetic material to cells. In neuronal cells, HSV establishes a latent infection without causing any adverse effect on the host cell. Consequently, it has become an attractive candidate for neuronal gene delivery.
Adenoviruses: Genomes persist in the cell nucleus predominantly as extrachromosomal episomes and they can only be used once because the immune system recognizes the virus and can provoke an immune response if used again.
Non-viral vectors: This class of vectors does not induce an immune response and provides a broad variety of choices. This includes liposomes, cationic polymers, lipoplexes and polyplexes, electroporation and many others. While they provide an improved toxicological profile, they suffer from less efficient delivery of the desired gene sequence.
Exhibit III - Types of Viral Vectors3
Click image to view larger
Lentivirus Vector
AGT has substantial experience with all types of vectors and has identified lentiviral vectors as the best vehicle to safely and effectively deliver its desired DNA sequence payload to the genome. All retroviruses (of which lentiviruses are a subtype) contain gag4, pol5 and env6 genes which code for viral proteins and the structure of the virus. Lentiviruses add two regulatory genes, tat and rev that enhance the efficiency of viral transcription and help to export mRNAs from the nucleus respectively. There may also be accessory genes that are involved in synthesis and processing of viral RNA. Lentiviruses contain the reverse transcriptase enzyme that converts RNA into DNA before integrating into the nuclear genome. Lentiviruses have the capability to transduce a wide variety of cells including stem cells and dividing or non-dividing cells.
In early versions of retrovirus vectors, integration often occurred near cancer-causing genes and the downstream LTR regions caused overexpression and tumor promotion. Newer versions of retroviral vectors and all of the current lentivirus vectors for clinical use turn off the ability of the terminal LTR to activate downstream gene expression, eliminating the risk of turning on something that should not have been. Lentivirus vectors rarely integrate near dangerous genes and they have proven safe for routine clinical use. Below, we follow the evolution of lentiviral vectors noting that AGT employs third generation versions which incorporate additional safety features and self-inactivation. Below, we highlight the key differentiating characteristics of each.
In early versions of retrovirus vectors, integration often occurred near cancer-causing genes and the downstream LTR regions caused overexpression and tumor promotion. Newer versions of retroviral vectors and all of the current lentivirus vectors for clinical use turn off the ability of the terminal LTR to activate downstream gene expression, eliminating the risk of turning on something that should not have been. Lentivirus vectors rarely integrate near dangerous genes and they have proven safe for routine clinical use. Below, we follow the evolution of lentiviral vectors noting that AGT employs third generation versions which incorporate additional safety features and self-inactivation. Below, we highlight the key differentiating characteristics of each.
First Generation
- Contains significant HIV genome (gag, pol & other viral proteins)
- Envelope protein is commonly the vesicular stomatitis virus G (VSV-G)
- Able to transduce a wide range of cells
- Improves the stability and broadens the cellular tropism of the viral particles produced
- Contains genes vif, vpr, vpu & nef
- Contains genes tat & rev
- Lacks sufficient safety controls for replication and survival
Second Generation
- Employs additional HIV proteins on plasmids in order to produce functional lentiviral particles
- Removal of accessory genes
- Express HIV gag, pol, rev and tat genes all from a packaging plasmid
- Envelope plasmid is interchangeable and usually encodes for VSV-G
- Replication incompetent and uses three separate plasmids encoding various HIV genes
- The long terminal repeat7 (LTR) viral promoter is wild type
Third Generation
- Express gag and pol from one packaging plasmid and rev from another
- Do not express tat
- Considered safer than second generation packaging systems
- May be more difficult to use vs. 2nd generation because they require transfection with four separate plasmids in order to create functional lentiviral particles.
- Transfer plasmid can be packaged by both 2nd and 3rd generation packaging systems
- Envelope plasmid is interchangeable and usually encodes for VSV-G
- Safety is improved over second generation
- Able to transduce a wide range of cells
- Self-inactivating – lack LTR sequences
- Replication incompetent
- Employs 4 plasmids (vs. 3)
- LTR viral promoter is a hybrid. 5’LTR is partially deleted and fused to a heterologous enhancer/promoter such as cytomegalovirus (CMV) or Rous sarcoma virus (RSV)
Benefits of Lentiviral Vectors
Lentiviral vectors:
- Address limitations of other approaches including AAV which have a cargo capacity of less than 5 kilobases (kb) while LVs can carry up to 10 kb.
- Are attractive for hepatic gene delivery as they stably integrate into the target cell genome and can potentially give rise to life-long expression of the therapeutic protein8.
- Exhibit low inflammatory response in vivo.
- Are the logical alternative to AAVs where 30-90% of individuals who have been exposed to AAVs have developed immunity (present antibodies or neutralize them).
AGT’s Lentiviral Vector Platform
AGT’s self-inactivating, third generation lentiviral vector construct is being used in its therapeutic programs. LVs do not express any potentially immunogenic viral genes or pro-inflammatory cytokines. AGT’s HIV program does not introduce vector directly into the body and there is no chance for direct toxicity from the viral vector.
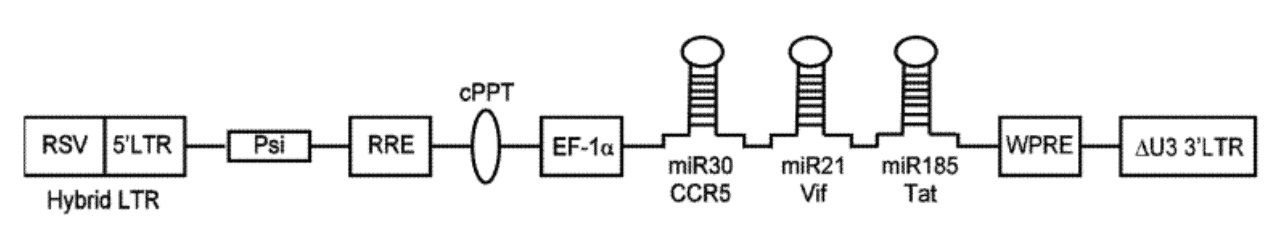
Exhibit IV - Linear Map of Lentiviral Vector9
Click image to view larger
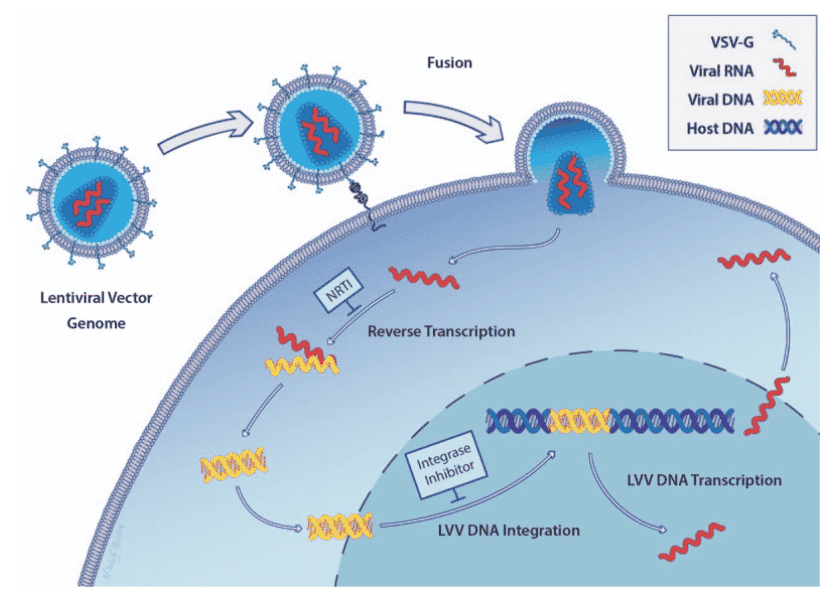
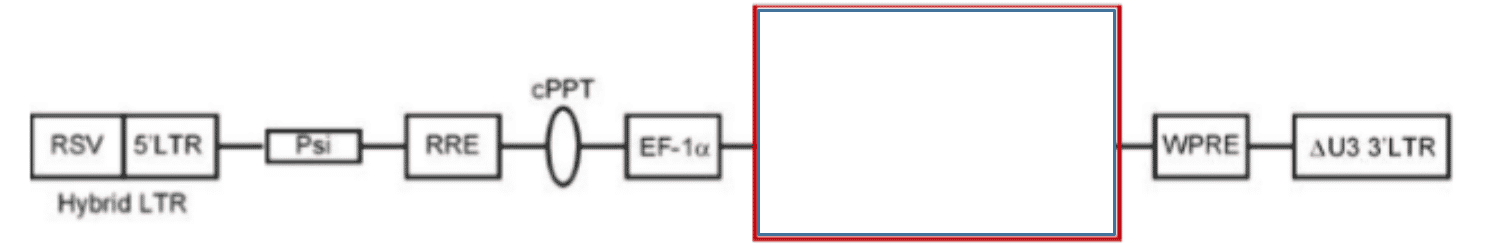
The preceding diagram illustrates a linear map of the LV system consisting of the following:
- RSV (Rous Sarcoma virus) long terminal repeat - a recombinant virus derived from RSV
- 5'LTR (long terminal repeats) - a portion of an HIV long terminal repeat that can be truncated to prevent replication of the vector after chromosomal integration
- Psi - a packaging signal that allows for incorporation of the vector RNA genome into viral particles during packaging ‣ RRE - a Rev Responsive element can be added to improve expression from the transgene by mobilizing RNA out of the nucleus and into the cytoplasm of cells
- cPPT - a Poly purine tract that facilitates second strand DNA synthesis prior to integration of the transgene into the host cell chromosome
- EF-1 Promoter of gene transcription - a promoter initiates RNA transcription from the integrated transgene to express micro-RNA clusters (or other genetic elements of the construct)
- Red box – genetic cargo which contains components to prevent expression of genes associated with the disease and decreases the spread of infection.
- WPRE - a woodchuck hepatitis virus post-transcriptional regulatory element is an additional vector component that can be used to facilitate RNA transport of the nucleus
- Delta U3 3'LTR - a modified version of a HIV 3' long terminal repeat where a portion of the U3 region has been deleted to improve safety of the vector
Risk Factors
There are a number of risk factors associated with gene therapy using viral vectors. We have provided a few examples above where four X-linked severe combined immunodeficiency (X-SCID) patients developed leukemia (insertional oncogenesis) when hematopoietic stem cells were transduced with a corrective transgene using a retrovirus. One of the main concerns relates to the new gene being inserted into the wrong location in the DNA. If inserted before an oncogene, leukemia or other oncogenic outcomes could develop. Another risk is that the new genes could be overexpressed creating an imbalance. In this circumstance there will be an excessive amount of the target protein. Additionally, the risks must be balanced in conjunction with the potential benefits of successful therapy which in many cases can provide a lifelong remedy to a previously chronic disease. AGT has addressed the risks addressed above and many others through its careful and refined approach based on its experience in viral vectors and review of extant literature. One example of AGT’s risk reduction therapy design is evident in the HIV program; only HIV-specific cells are modified, allowing the important CCR5 co-receptor to remain on non-HIV-specific cells, protecting the patient from a vast array other diseases. Another example is evident in the oncology indication, where the genetic modification does not affect the T cells, but rather the tumor cells, avoiding a change in the constituents of the immune system. This allows T cells to assume their normal function and eradicate the cancer while returning to normal once the tumor is resolved. The PKU vector also has safety advancements; when synthetic promoter/enhancer regions are built for liver-specific gene expression, they have a high degree of similarity to normal liver genes and they express with a normal pattern and avoid abnormally high expression levels. The LV vector also can use much lower particle doses than AAV approaches. The difference may be a factor of 10,000, substantially reducing the level of foreign material used in the patient.
Pipeline
The human immune system has a big job. It needs to identify foreign matter that enters the body and attack and remove it. The foreign matter can be bacteria, viruses, fungi and toxins and the role of the innate and acquired immune system is to recognize these invaders and eliminate them. Sometimes our systems fail to clear dangerous attackers and they can infiltrate the body. Some viruses, such as human immunodeficiency virus (HIV) and hepatitis C are able to infect critical cells and lay dormant for many years and in most people, the immune system lacks the natural ability to clear the virus. Cancers are another area where an imbalanced immune system may fail to stop the disease. Cancer cells have many mechanisms to avoid detection, including the expression of checkpoints or the lack of sufficient differentiation from normal cells for the immune system to recognize them. To address this shortcoming, various types of immunotherapy have been developed. Immunotherapies can either be activation therapies, which increase sensitivity or suppression therapies, which dampen the immune system.
Some of the leading approaches in this field that have distinguished themselves include:
- Redirected T cell responses (CAR-T)
- Monoclonal antibodies (mAbs)
- Oncolytic viruses (Talimogene Laherparepvec)
- AGT’s ImmunoTox
While the most visible immunotherapies are focused on cancer, due to the prevalence of the disease and frequently deadly outcome, immunotherapy can be used in a wide variety of conditions such as rheumatoid arthritis, allergies, inflammation, diabetes and cardiovascular diseases among many others.
AGT has developed its current pipeline based on identifying diseases that are conducive to gene therapy treatment, that are very expensive to treat and where there is no cure. These constraints have yielded candidates in three different indications: HIV, PKU and cancer, all of which can be efficiently addressed with viral vectors and have a high probability of success.
Human Immunodeficiency Virus (HIV)
Human Immunodeficiency Virus (HIV) and acquired immunodeficiency syndrome (AIDS) was first mentioned in 1982 in a New York Times article speculating on the cause and origin of a mysterious disease. In the months prior to the article, there were several unusual outbreaks of pneumonia, Kaposi’s Sarcoma and fatal illnesses from what in a normal immune system is considered harmless bacteria. The research found that the disease was prevalent in homosexual men and in IV drug users among other populations. As the 1980s progressed, the disease began to receive resources from the government to slow its spread. Testing for its presence and education regarding transmission, mechanisms to protect oneself and knowledge of HIV was disseminated, helping reduce infection rates. As research continued and new therapies launched, the death rate from the disease slowed in the latter half of the 1990s. Based on research using viral family tree ancestry it is speculated that the virus had been transmitted from chimpanzees to humans sometime early in the 20th century. Additional research published in Science provided a remarkably detailed pathway of virus dissemination from the Cameroon in the 1920s to spread to the wider world over the following decades.
According to UNAIDS, there were about 37 million people globally with HIV/AIDS in 2017 and according to the CDC, 1.1 million in the U.S. in 2015. While the number of people dying from the disease has slowed since the advent of antiretroviral therapy, there were still almost 16,000 deaths in 2016 for those diagnosed with it. Over the history of HIV/AIDS, approximately 70 million10 people have been infected with HIV and half of them have died.
How Does HIV Work?
HIV is an infection that damages the immune system and causes AIDS. The virus is passed from person to person through bodily fluids such as blood, semen, rectal fluids, vaginal fluids and breast milk. The fluids must come into contact with the mucous membrane or enter the bloodstream usually through needle drug use.
Exhibit V - Human Immunodeficiency Virus (HIV)11
Click image to view larger
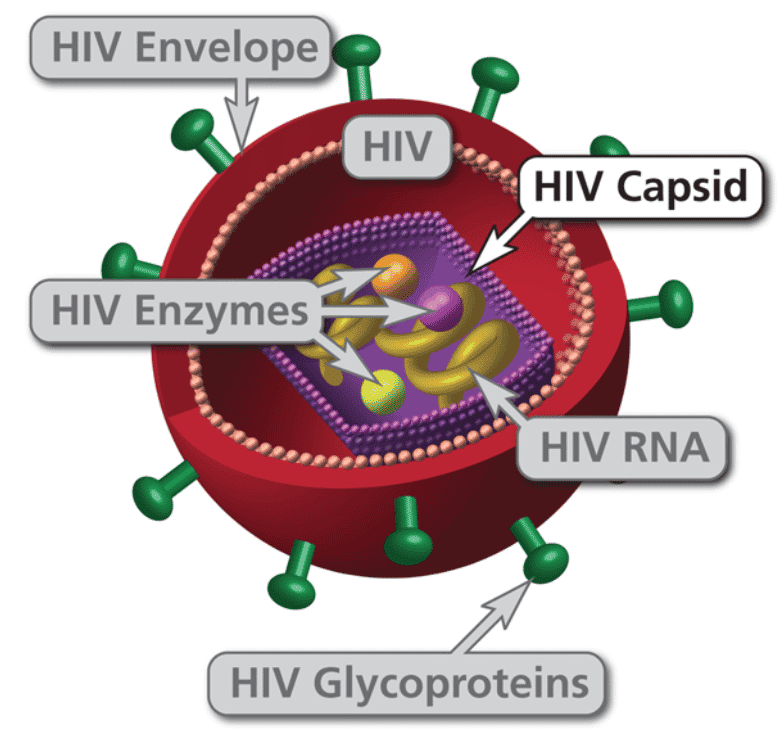
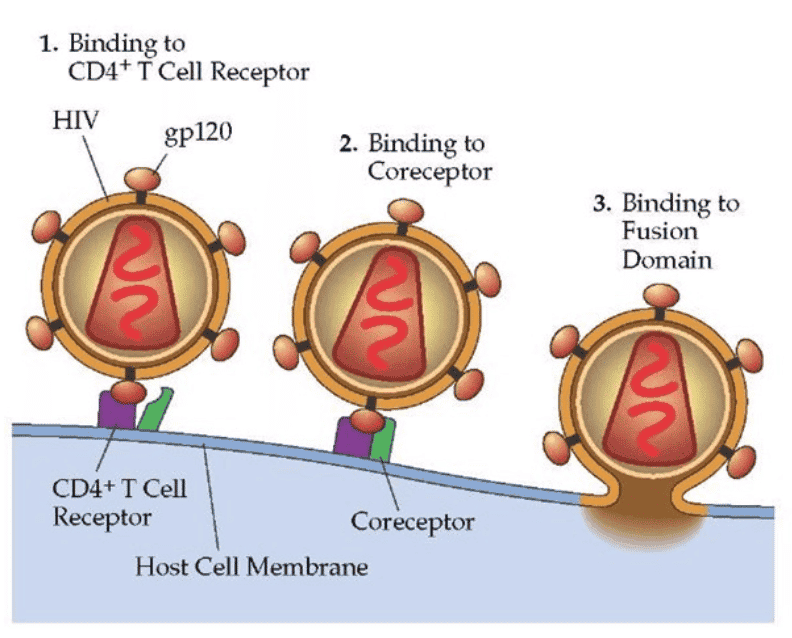
HIV is a retrovirus with two copies of RNA, reverse transcriptase, integrase and protease among other proteins contained inside the capsid. The virus targets CD4+ T cells (T helper cells) through the CD4+ receptor on the surface of the T cell with the HIV surface protein, Gp120. After the CD4+ receptor binds to the HIV virion, then a second receptor called chemokine co-receptor (CCR5)12 grabs hold of the envelope and draws it to the surface of the CD4+ cell. The stalk of the virion then pierces the surface of the CD4+ cell and fuses the viral membrane and the CD4+ membrane together. The material in the virus is then injected into the cell.
Exhibit VI - HIV Binding to CD4+ and Co-receptor on T Cell13
Click image to view larger
After the viral capsid enters the cell, viral RNA and enzymes are released. Reverse transcriptase takes over and converts the single strand of RNA into a single strand of DNA using host nucleotides, then it synthesizes another complementary strand of DNA creating a double stranded form of viral DNA. Reverse transcriptase is error prone during its polymerization process creating many mutations in the viral DNA, which can confer resistance to some antiviral medications and can also render vaccines ineffective. After the conversion to double stranded DNA is complete, then the integrase enzyme shuttles the DNA into the nucleus where it finds an appropriate site in host cell DNA and inserts the viral DNA into the chromosome. See here14 for a video presentation illustrating the process.
This process creates the lifelong infection in the CD4+ cell. If the viral DNA is not transcribed into messenger RNA (mRNA), then it is considered a latent infection. However, if the DNA transcription enzyme transcribes that section of the DNA, then the mRNA will be activated and exported from the nucleus to find ribosomes where the mRNA translates envelope protein. Other viral proteins (polyproteins) are also generated which eventually become reverse transcriptase, integrase and extra viral proteins that are needed for the virion to later become infectious. Along with the envelope protein, polyproteins accumulate on the surface of the CD4+ cell to create a new HIV particle. As the new HIV particle begins to bud off, a protease cleaves the polyprotein into its component parts. After the proteins are all successfully cleaved into the components of a new viral cell, it now becomes mature and leaves the CD4+ cell to infect other CD4+ cells.
The HIV-specific T cells are not only infected by HIV but are simultaneously trying to fight it. The HIV virus activates T cells and they begin to expand at a high rate during infection at the same time the HIV is replicating and spreading throughout the body. This leads to a huge number of infected HIV-specific T cells with up to 10 x 109 viral particles per mL of plasma. Following the acute infection, the number of cells declines dramatically through apoptosis and pyroptosis and virus levels decline as well to approximately 10 x 104 viral particles per mL of plasma. This level characterizes chronic HIV infection and the objective of antiretroviral drug therapy is to suppress it to an even greater degree.
Following the acute infection phase, which lasts from one to three months, a period of clinical latency ensues, resulting in slow disease progression that can last from several years to over a decade. After this period of latency ends, an event usually brought on by stress or exposure to opportunistic infection or disease combined with the constant replication of HIV, which was ongoing during the clinical latency, degrades host immunity to a failure point. The body then lacks the ability to fight infections and a broad array of illnesses and infections lead to AIDS and eventually death of the host.
Diagnosis
Blood tests are generally used to diagnose HIV by identifying antibodies that signal the presence of the disease. It does take some time for the body to develop the antibodies that the test recognizes, usually six or more weeks. One example of this test is the enzyme-linked immunosorbent assay (ELISA). Confirmation of an ELISA is performed with the Western blot test. Viral load tests are also performed on blood samples and measure reverse transcription polymerase chain reaction (RT-PCR), branched DNA (bDNA) and nucleic acid sequence-based amplification assay (NASBA). An antigen test may also be used and can give an earlier response as antigens are produced immediately after the infection.
Treatment
There is no cure for HIV/AIDS; however, there are several classes of treatment. They include non-nucleoside reverse transcriptase inhibitors (NNRTIs) and nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs) which neutralize the enzyme that creates a copy of the viral RNA. Protease inhibitors, entry or fusion inhibitors and integrase inhibitors are other approaches that disrupt the viral construction process inside the target cell.
A combination of these drugs is used in treatment regimens called antiretroviral therapy (ART) or highly active antiretroviral therapy (HAART). The combination approach has been successful and has maintained low viral levels preventing transmission. The use of ART has converted treatment into a type of prevention as well, leading to the development of pre-exposure prophylaxis (PrEP) therapy. PrEP therapy is intended and appropriate for high risk populations that do not have HIV but may become exposed to it. It is a combination of antiretrovirals branded Truvada or one of many emerging drug combinations. The regimen reduces the probability of contracting HIV by 90% for those in this category if persons maintain strict and uninterrupted compliance to the treatment schedule.
As good as antiretrovirals are, they cannot eliminate the entire virus and if the virus mutates, it could evolve into a new form that is not treatable. To address this difficulty, the patient must use a combination or cocktail of drugs to permanently suppress the virus from multiple directions. Patients on HIV treatment must take the drug for the rest of their life, which is burdensome, side effect prone and expensive.
Therapies Under Development
Sangamo Efforts
During the early part of the current decade, Sangamo Therapeutics used an ex vivo gene editing approach with an adenovirus vector to disrupt expression of the CCR5 co-receptor. There was a therapeutic effect in some patients, but it did not cure the majority of the patients in the trial. It is hypothesized that insufficient amounts of the modified gene were penetrating the cells that needed it and the approach did not control pre-existing HIV or modify infection by HIV strains using other co-receptors for cell penetration, preventing a cure from taking place.
Other cures in development focus on therapeutic vaccines, integrase inhibitors, capsid inhibitors, CCR5 antagonists (cenicriviroc), rev inhibitors, non-nucleoside reverse transcriptase inhibitors, gp120 attachment inhibitors and others.
AGT103-T
American Gene Technology’s lead asset is AGT103-T15, an ex vivo, autologous cell therapy product targeting HIV infection. The drug is composed of a viral vector and a DNA payload which protects CD4+ T cells from attack by HIV by downregulating the CCR5 receptor and inactivating viral RNA. The lentivirus vector is delivered to CD4 T cell highly enriched for an HIV-specific subset. The lentivirus vector prevents destruction of these HIV-specific T cells which are critical to eradicating HIV from the system.
The treatment is designed to improve the ability of the immune system to recognize infected cells and destroy them, thereby eliminating them from the body. AGT103 is a third generation, self-inactivating lentivirus vector that carries three inhibitory RNA embedded in a microRNA backbone. Each of the three miRNA have a role in eliminating HIV and disrupt the viral RNA from propagating within infected cells. Precise lentivirus vector targeting of gag-specific CD4+ T cells allows other T cells to continue their sentry efforts required in other immune responses.
AGT103-T Preparation
AGT103-T therapy is similar to what is performed in the well-publicized chimeric antigen receptor T (CAR-T) cell therapy, which is a multi-step process that withdraws, modifies and reinfuses cells into a patient’s body. Below we summarize the general process.
1. T cells are removed through a blood draw (apheresis or leukapheresis)
2. Withdrawn cells are taken to a manufacturing site (HCATS)16
3. Cells are modified using a lentivirus vector
4. Modified cells are returned to the patient
Exhibit VII - Therapeutic Lentiviral Vector with Helper & Envelope Plasmid17
Click image to view larger
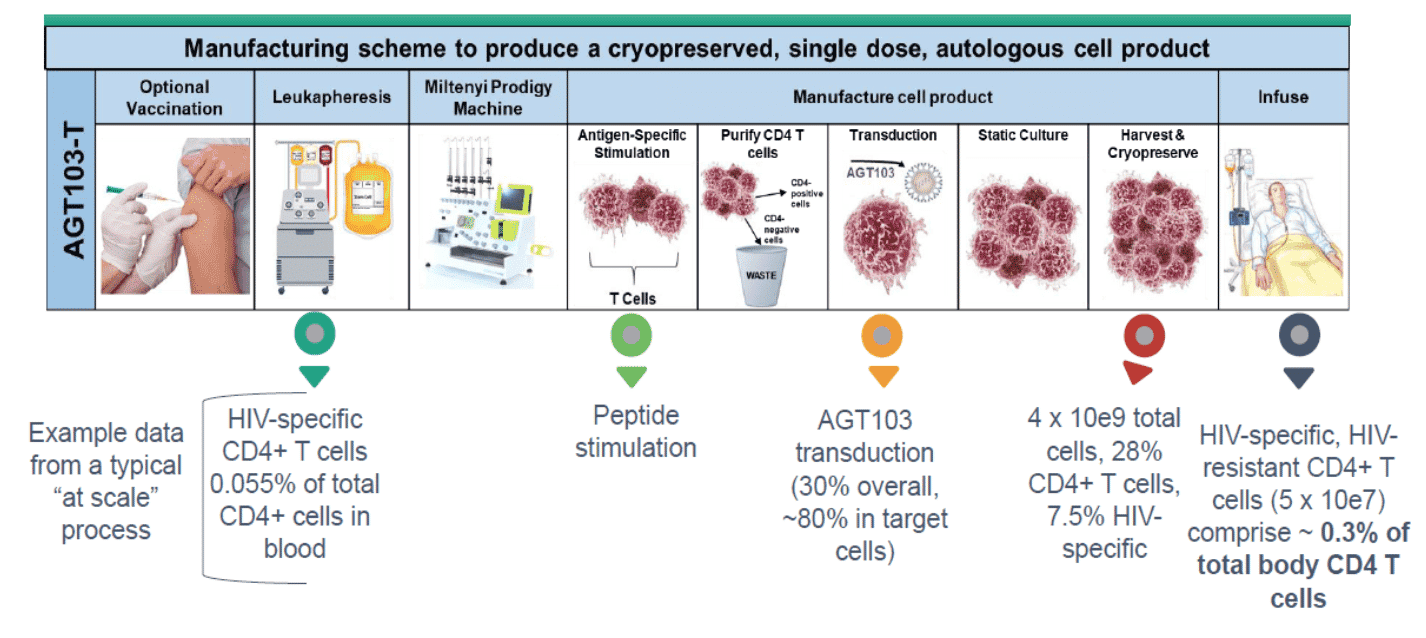
The method of treating HIV infection may or may not include pretreatment with a vaccine, which is intended to enrich or prime the cells and increase the frequency of HIV-specific CD4+ T cells. Leukocytes are then removed from the patient via leukapheresis to obtain peripheral blood mononuclear cells (PBMCs). The HIV-specific T cells are transduced in an automated cell processor where they are exposed to gag specific antigens to train the T cells to target HIV, then cells are purified to isolate CD4+ cells. The PBMCs which include HIV-specific CD4+ T cells are transduced ex vivo using the lentiviral viral vector delivery system which encodes for several features including downregulating the chemokine receptor CCR5 and small RNA capable of targeting an HIV RNA sequence. After culturing and expansion, the cells are harvested, cryopreserved and shipped to the patient. The literature suggests that a minimum of 1 x 107 transduced, HIV-specific CD4+ T cells are required to induce a memory immune response; however, the precise level is not known and will vary based on the individual. As part of the protocol development, this count will be determined and infused into the patient to begin AGT103 therapy. In most AGT103-T cell products, the levels of HIV-specific CD4+ T cells exceed the minimum number by a factor of 10x to 50x.
Exhibit VIII - AGT103-T Manufacturing Process18
Click image to view larger
The approach to cure HIV recognizes that the cells best equipped to eradicate HIV from the body are also the ones that are targeted and killed first by the virus. If an HIV positive individual begins ART, their CD4+ T cells will begin to repopulate and provide the necessary material to use in the ex vivo lentiviral transduction process.
Mechanism of Action
Patients must have one to three years of ART and maintain control of the HIV disease to be eligible for treatment. Control is measured as undetectable viral RNA, stable CD4+ T cell counts above 500 cells/mm3 and no AIDS defining condition. This ensures that the suppression of HIV has been of adequate duration so that sufficient numbers of HIV-specific CD4+ T cells are available to receive the ex vivo treatment. As the transduced cells are infused into the body, they have downregulated CCR5 co-receptors which will prevent HIV infection. Several different micro RNA (miRNA) are also introduced into infected cells. One miRNA sequence halts the production of HIV proteins by binding to and destroying the messenger RNA that would have produced viral proteins. Other miRNA can downregulate the expression of CCR5. When HIV-infected cells are transduced during the ex vivo process, they too will shut down the RNA transcription process. AGT103-T attacks the HIV from multiple directions. Transduced cells will proliferate and direct normal anti-viral activity thereby clearing the virus from the body.
The AGT103-T cell manufacturing process generates up to 1 x 1010 CD4 T cells enriched for HIV-specific T cells that are transduced with the AGT103 vector. This process delivers HIV-specific CD4 T cells at much higher levels than were used used in the Sangamo studies and addresses one of their major shortcomings, which was insufficient therapeutic effect. While Sangamo sought to block HIV replication by broad reductions in CCR5 levels, AGT103-T is a true immunotherapy seeking to reconstitute a natural capacity for eradicating the virus.
Safety
The FDA has a strong grasp of all the key components that comprise AGT’s efforts in HIV. The modification and expansion process are well understood from the two approved CAR-T therapies that exist in the market. Viral vectors are also well understood and the oncogenic and immune responses that caused severe side effects 20 years ago have been addressed and refined with later generation viral vectors that increase safety and minimize inflammatory response in patients. The AGT103-T therapy also avoids adding features to the transduced cells and rather subtracts or downregulates the CCR5 receptor. The process also focuses on a small subset of immune cells, allowing other CD4+, non-HIV-specific cells to persist unmodified. The number of cells is similar to doses being reintroduced into the body in CAR-T therapy for example. These features and the growing experience with modified T cell products for therapy have simplified development and testing of AGT103-T.
Preclinical Work
Eleven cell process runs have been completed at clinical scale and the AGT103-T process is robust and reproducible. Modified cells resist HIV infection. Process development was completed in part, with clinical specimens obtained from HIV-infected volunteers participating in AGT’s CS-168 study.
AGT is currently conducting IND-enabling work and plans an IND submission in 2019. Several requirements have been completed so far including cGMP vector manufacturing and an animal toxicology study using AGT103-T product from pilot runs of the final cell process. There remain several outstanding items to complete for the IND application including final engineering and process qualification activities at the cell manufacturing partner and completion of process qualification. Cell process and development and testing is being conducted in preparation for IND submission which is being targeted for the second half of 2019. As per FDA protocol, 30 days following the submission of the IND, AGT may begin the trial if the agency has no concerns.
Phase I Clinical Trial
A Phase I clinical trial is planned to begin in fourth quarter 2019. The study will examine the safety and feasibility of AGT103-T in 18 subjects and is expected to take 18 months to complete. Costs of the trial are estimated to be approximately $7.5 million, including cell product manufacturing, clinical testing, clinical services and data analysis.
Phase II
Assuming a successful Phase I study, a Phase II study will be designed to enroll sufficient participants to power the trial and measure effectiveness and safety on a larger scale. One of the hurdles that the company recognizes is the increasing demand on cell product manufacturing resources arising from growth of CAR-T and virus specific T cell therapies. It is likely that the company will be able to bank sufficient vector and payload assets in advance of the trial. Hitachi Chemical Advanced Therapeutics Solutions (HCATS) has contracted with AGT to provide transduced cells for its preclinical work and we anticipate the relationship will continue throughout the development process.
The Phase II plan will be broader than simply conducting the trial. Training for HIV care providers to familiarize them with gene therapy will be organized. This is especially important due to side effects that can be experienced by patients who receive modified cells including cytokine response syndrome or immunologic inflammatory syndrome. Community relations will also be a component of Phase II preparation. Advocacy from groups most exposed to HIV will be paramount as their efforts to populate the trial will be critical.
Phase III
Phase III trial design will be based on the outcomes in earlier studies. AGT103-T may receive expedited treatment and successful data from a Phase II trial may be sufficient to receive accelerated approval. In the case of accelerated approval, post-marketing studies will be required.
Costs of Manufacture
Demand for cell processing has been strong in recent years as CAR-T and other ex vivo processes have grown. Based on the current environment, AGT estimates that manufacturing costs will be between $5 and $10 million per year in the research phase and rise to $100 million or more during the commercial stage.
HIV Summary
HIV was one of the most frightening diseases when it was first discovered and generally thought to be a death sentence until antiretroviral therapy was developed and made available. While progress has been made to treat HIV and AIDS a cure has not yet been developed, which leaves millions of individuals with the disease tethered to their ART. ART extends life but also includes severe side effects. The cost is also high for a lifetime of expensive therapy, demonstrating the importance of finding a cure that will allow health care resources to be used elsewhere. AGT103-T addresses the HIV infection by first preventing the virus from gaining access to CD4+ T cells then by employing miRNA to neutralize the impact of viral proteins being created by infected CD4+ T cells. The multi-pronged approach is expected to eradicate HIV from the system and return the patient to a healthy state. While we are still in the early stages of development, an IND and start of a Phase I trial are expected this year.
Liver Cancer
The human liver filters foreign agents from the gastrointestinal tract and has intrinsic mechanisms to prevent a broad immune activation that is otherwise triggered by antigens from the portal vein. However, this innate tolerance is unbiased as it could potentially fail to respond to tumor-associated antigens (TAAs) and other stimulants leading to Hepatocellular carcinoma (HCC) growth and progression. Additionally, most cases of HCC occur from chronic liver inflammation that promotes immune suppression. Due to these immunosuppressive mechanisms involving several mediators and suppressive molecules, most HCC patients remain refractory to therapy.
HCC is an aggressive form of primary liver cancer. A chronically inflamed liver ultimately results in cirrhosis and poses as a major risk factor for developing HCC. Patients with liver cirrhosis frequently suffer from immune dysfunction. HCC is most often diagnosed late in its course and its general prognosis remains poor. The median survival time following diagnosis is approximately 6 to 20 months and the five-year survival rate is about 19%19. As per CDC statistics, it is the third leading cause of cancer death globally. According to the World Health Organization, the global incidence of HCC is estimated to be over 750,000 annually.
Treatment
Cancer therapy involves destroying all cancer cells, including those that may be in a precancerous stage, while sparing normal cells. Treatment options for patients with carcinomas metastatic to the liver are targeted therapy, ablation and/or liver transplant that help in prolonging life for a few months. However, they are limited to a select subset of patients that have early stage metastasis or few small tumors or tumors that can be detected radiographically. Surgery is performed only in those patients whose tumors have not grown into blood vessels or whose liver has good functionality despite the tumor; and this is true in less than 20% of patients. Surgical resection remains the first line therapy in patients having small tumors and who have preserved liver function and no underlying/slight cirrhosis. Orthotopic liver transplantation (OLT) remains the mainstay therapy for a small percentage of HCC patients with moderate to severe cirrhosis. If diagnosed early, OLT offers a potential curative therapy, with 5-year overall survival of 75% and a tumor recurrence rate of less than 15% 20, 21, 22.
There is evidence in literature that once the cancer has metastasized to the liver, the median overall survival for the patient is about six to eight months23. For micro-metastases in the liver that cannot be detected using CT scan, a loco-regional therapy such as radiofrequency ablation (RFA), trans-arterial chemo-embolization (TACE), percutaneous ethanol ablation or radioembolization is an option but only provides palliative treatment. In chemoembolization, chemotherapy and embolic agents are passed through a blood vessel that feeds the cancerous tumor to cut off the tumor's blood supply. TACE is currently the established therapy for both primary and secondary hepatic malignancies. Selective Internal Radiation Therapy (SIRT) is a radioembolization procedure that employs radioactive microspheres via the hepatic artery to target liver tumors. Therefore, exposure of radioactive substance to the remaining healthy liver tissue is minimized.
The current standard of care for advanced HCC is sorafenib (Nexavar by Bayer Pharmaceuticals), a kinase inhibitor, was approved by the FDA in 2007 for advanced renal cell carcinoma and unresectable HCC. Approval was based upon the results of two large randomized, double-blind, placebo-controlled, Phase III trials (SHARP and Asia-Pacific). Both trials demonstrated that at the dose of 400 mg given twice-daily, sorafenib significantly extended overall survival by 2 – 3 months in HCC patients who remained refractory to loco-regional procedures. For patients who are refractory to sorafenib, the FDA approved regorafenib (Stivarga by Bayer Pharmaceuticals) in 2017, nearly a decade later. Approval was based on the outcome of a Phase III clinical trial of 573 patients with HCC that had progressed after treatment with sorafenib (Bruix et al., 2017). Regorafenib extended median overall survival by 2.8 months compared to placebo.
Therapies under investigation
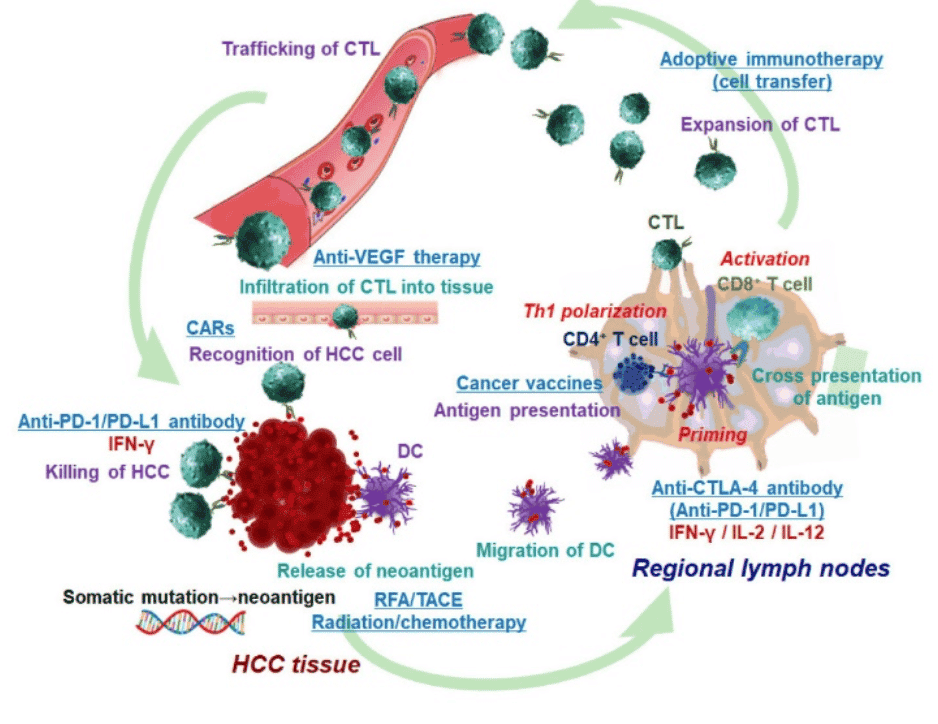
HCC cells produce various TAAs and neoantigens. The initial anti-tumor immune response includes uptake of TAAs and neoantigens by dendric cells (DCs). DCs then migrate into regional lymph nodes and present processed antigen to CD4+ and CD8+ T cells. Antigen recognition leads to proliferation of CD4+ T cells and induction of interferon (IFN)-γ in the presence of IL-12 and type I IFN (Th1 polarization). Antigen-specific CD8+ cytotoxic T lymphocytes (CTLs) are developed. The antigen-specific CTLs exert anti-tumor function. Below we discuss the various treatment approaches that target the different stages of cancer progression.
- Cell therapy: Cancer vaccines promote antigen presentation; anti-CTLA-4 antibody mainly acts in priming phase and facilitates the Th1 polarization and activation of CD8+ T cells. DC vaccines are capable of inducing and activating the effector anti-tumor CTLs. However, the immune system of most advanced cancer patients cannot be activated or may only be activated to a limited extent by DCs. In such cases, DCs are cultured and expanded ex vivo for immunotherapy24. In general, therapeutic efficacy of cancer vaccine is limited by a dearth of tumor specific antigens, immunosuppressive effect of tumor microenvironment and tumor heterogeneity.
- Adoptive immune therapy: This consists of transferring a large number of CTLs with T cell receptors recognizing specifically TAAs and/or other cytotoxic cells like NK cells into patients. Autologous cells obtained from patients are expanded ex vivo and adoptively transferred after genetic modifications25.
- Chimeric antigen receptors (CAR) therapy: T-cells work independently of antigen processing and presentation. In CAR-T-cells, an antibody joins TCRs to recognize cell surface antigens in an MHC-unrestricted approach. These can be manipulated to specifically target malignancy-associated antigens. Different types of antigens can be used as potential targets, such as tissue-specific differentiation antigens, germ cell antigens, overexpression of self-proteins, mutational antigens and viral antigens. CAR-T has already been applied to treat some solid tumor and homological malignancies26. CAR-modified T cells can be developed to target any tumor antigen; however, specificity for tumor rather than host antigens is of paramount importance with regard to limiting toxicity.
- Anti-vascular endothelial growth factor (VEGF) therapy: Anti-VEGF therapy potentially induces infiltration of T cell into tumor tissues and inhibits activation of downstream multikinases that are normally essential for cell growth, angiogenesis, proliferation and metastasis of HCC cells. Anti-VEGF therapy with sorafenib has been successful as the first systemic therapy and has demonstrated improved survival in patients with advanced-stage HCC27.
- Checkpoint inhibitor therapy: Therapy using immune checkpoint inhibitors is established on the role of T-cell mediated immunosuppression through immune checkpoints. Activation of T-cells by DCs is through ligation of MHC class I/II and T-cell receptor (signal 1) and costimulatory molecular pathways (signal 2). Costimulatory molecular pathways may deliver positive or negative signals to T-cells and result in T-cell activation or T-cell anergy (functionally inactive) to specific antigens. Checkpoint inhibitors can block negative costimulatory molecular pathways and enhance T-cell-mediated immunity. Blocking the immune checkpoint molecules (CTLA-4) restores T cell function, allowing the immune system to more effectively detect and kill the HCC tumor. Only immune checkpoint therapy using an anti-PD-1 antibody has produced favorable outcomes in clinical trials. Nivolumab, a monoclonal antibody to block programmed cell death protein 1 (PD-1), showed high efficacy in patients refractory to sorafenib. Immunotherapy by checkpoint inhibition can cause immune-related adverse events (irAEs) in a considerable number of patients due to the induction of overstimulation of immune reactivity or to the generation of outright autoimmune phenomena28.
Exhibit IX - Immune System in Cancer29
Click image to view larger
Immunotherapy for advanced HCC patients remains controversial as it lacks sufficient efficacy in preventing recurrence and prolonging survival. Therapies have largely been confined to preclinical and experimental settings and have provided divergent results.
The role of γδ T cells in immune-surveillance and immune defense against tumors
About 5% of circulating lymphocytes are made from a smaller subset of T-cells expressing T cell receptors (TCRs) made from γ and δ chains. These are termed γδ (gamma Delta) T cells whose functional response is triggered by the presence of antigens. They have the capability to attack infected cells indirectly by activation of other immune cells. They promote cytokine production without the presence of major histocompatibility complex (MHC) molecules and manage clearance of foreign bodies, control inflammation and regulate tissue homeostasis. γδ T cells multiply at high frequencies which allows them to respond to tumors and pathogens. The advantage of using γδ T cells is that they are abundantly available in the peripheral blood. Additionally, γδ T cells selectively kill cancer cells without affecting the non-stressed (non-transformed) cells. Also, they could be cultured ex vivo or stimulated and expanded in vivo30, 31. All these features make them excellent candidates for cancer immunotherapy.
The mevalonate (isoprenoid) pathway is an integral metabolic pathway in the cellular processes associated with tumor formation. Prenyl pyrophosphates are intermediates in the series of enzymatic reactions in isoprenoid biosynthesis and the γδ T cells monitor these elements in isoprenoid metabolism. Accumulation of metabolites from the mevalonate pathway is a red flag that activates the γδ T cells. Under normal conditions, isopentenyl pyrophosphate (IPP), a downstream signal produced by mevalonate pathway, is at a low concentration that does not elicit a γδ T cell response. Any dysregulation of the mevalonate pathway results in IPP production in high concentrations. γδ T cells are able to recognize and kill various tumor cells, either spontaneously or after treatment with zoledronic acid (ZA)32, 33, 34. ZA is currently the most potent nitrogen-containing bisphosphonate, which has shown potential as an immunotherapeutic drug for cancer35.
LV ImmunoTox
AGT is developing a lentiviral vector, ImmunoTox, which modifies tumor cells to trigger the anti-tumor response of γδ T cells. The activated T cells target both genetically-modified and unmodified tumor cells. AGT’s approach triggers the γδ T cells to invade cancer at the site of the treated primary tumor as well as against metastases. γδ T cells have not been linked to auto-immunity.
γδ T cell receptors are known to respond to nonpeptide prenyl pyrophosphates. This detection is key in microbial and tumor immunity. Farnesyl Diphosphate Synthase (FDPS) is a branch point enzyme in the synthesis of sterols and isoprenylated cellular metabolites mainly known to mediate immunoregulatory functions. Inhibition of FDPS in tumor cells using ImmunoTox, blocks isoprenoid metabolism resulting in IPP accumulation. Small increases in IPP levels are recognized by γδ T cells causing them to secrete high levels of inflammatory cytokines to lyse tumor cells.
Cancer research has been taking advantage of the humanized mouse model (immunodeficient NOD-scid IL2rc null (NSG) mice) to better understand the mechanisms of cancer and develop T cell-based immunotherapy approaches. Peripheral blood mononuclear cells (PBMCs) are of particular interest in humanized mouse models since they engraft immunodeficient mice with mature T cells in about a span of one week that are suitable for short term studies.
Mice with spontaneous liver tumors were systemically injected via the tail vein with AGT’s lentiviral vector expressing firefly luciferase proteins. Imaging performed as part of the analysis presents bioluminescent markers in harvested livers five days post injection. Preferential transduction of liver produced higher light emission showing the ability of the lentiviral vector to target and transduce tumors after injection.
The mice were randomly divided into four groups of eight mice each. One group was treated with Zoledronic acid. Research has shown that systemic ZA can increase phosphoantigen expression in subcutaneously implanted tumors and so facilitate γδ cell-mediated tumor killing in humanized mice36. In the untreated group, overall survival dropped after 60 days. In the group treated with PBMC alone, overall survival was at 75% 90 days post-transplant. The PBMC+ZA group had the best overall survival of about 80%.
AGT’s candidate LV-401 slows tumor growth. Results demonstrated that LV-401 with PBMC transplantation/ injection exhibited a marked inhibitory effect on tumor growth. Studies have shown that PBMC transplantation results in reconstituting the immune system37. The group treated with LV-401 + PBMC had a survival of greater than 75% at day 110 after tumor implantation. ZA was not required for increased survival rate.
Is ImmunoTox better than other approaches for treating HCC?
- CAR-T therapy primarily targets hematologic malignancies. Solid tumors have been challenging to treat with CAR T-cell therapy due to the following reasons:
- Identifying unique cell surface markers that distinguish solid tumor cells from normal tissues is difficult.
- A solid tumor’s external matrix structure and its highly immunosuppressive nature do not allow CAR T-cells to communicate with infected cells effectively.
- However, several studies are investigating the potential of CAR-T therapy in solid tumors. γδ T cell response, triggered by ImmunoTox, does not require unique cell surface markers for specific tumors, as is required by CAR-T or monoclonal antibody cell therapy.
- Studies have shown that γδ T cells appear less sensitive to immune checkpoint inhibitors compared to other T cells. Targeting γδ T cells using LV is a promising alternative as LVs can be produced by a minimal set of viral genes.
- Further, ImmunoTox is a SIN vector that decreases the risk of vector mobilization and recombination.
- Additionally, ImmunoTox provides for local stimulation of γδ T cells that elicits a more targeted response with less systemic exposure to therapeutic agents.
- γδ T cells do not recognize antigens restricted by MHC molecules which makes it possible for autologous as well as allogenic therapy to be developed.
Market
A majority of primary liver cancers are HCC. While targeted therapies for HCC offer a modest overall survival benefit, immunotherapy promises an increased potential benefit including progression-free survival. Nevertheless, despite the therapeutic benefit offered by checkpoint inhibitors, currently available treatments do not offer a cure.
Although great strides have been made in treatment, when examining the pipeline of potential candidates, a number of oncology drugs in clinical trials have failed to show efficacy. In 2010, Pfizer discontinued a Phase III study of Sutent (sunitinib malate) as it caused serious adverse events and failed to prove superiority to sorafenib. In 2012, Bristol-Myers Squibb’s molecule brivanib failed to meet the primary endpoint of non-inferiority to Nexavar as a first-line treatment for HCC. In 2014, Novartis’ Afinitor (everolimus) did not improve overall survival in patients with advanced HCC who were refractory to sorafenib.
A search for “advanced HCC” and “Phase III studies” in the NIH U.S. National Library of Medicine web-based registry of clinical trials38 yielded 21 results of ongoing investigations of drug candidates or combination therapies. Earlier this year, Eli Lilly announced results of a Phase III REACH-2 study of CYRAMZA (ramucirumab) as a single agent in the second-line treatment of people with AFP-High (alpha-fetoprotein ≥400 ng/mL) HCC. Patients in the REACH-2 and REACH trials demonstrated an improvement of 3.1 months in median overall survival. Celsion’s ThermoDox’s pivotal 556-patient global Phase III OPTIMA Study in HCC completed enrollment in August 2018 and the first interim efficacy analyses is expected in the second half of 2019.
A billion-dollar unmet medical need in the immuno-oncology space presents a compelling market opportunity for AGT. As per a new report by Grand View Research in 2018, the global liver cancer therapeutic market is expected to grow at a CAGR of 19% driven primarily by the increase in patient volume. The HCC therapeutic market is currently dominated by Nexavar (sorafenib by Bayer) followed by other second line of treatments Opdivo (nivolumab by Bristol-Myers Squibb) and Stivarga (regorafenib also from Bayer) and off-label chemotherapies. Sales of Nexavar reached close to a billion dollars in 2017. The wholesale price of Stivarga is roughly $15,000 for one course of treatment. Opdivo, a checkpoint inhibitor, was approved for patients who are previously treated with Nexavar. Opdivo’s U.S. revenues reached north of $1 billion in 2018 and is priced from $150,000 to $165,000 per course of treatment. Merck’s cancer drug Keytruda, which is priced similarly, failed both overall survival as well as progression-free survival in a Phase III study in advanced HCC. Currently, there are several ongoing trials investigating Keytruda in combination with other treatments in patients with advanced HCC.
Phenylketonuria
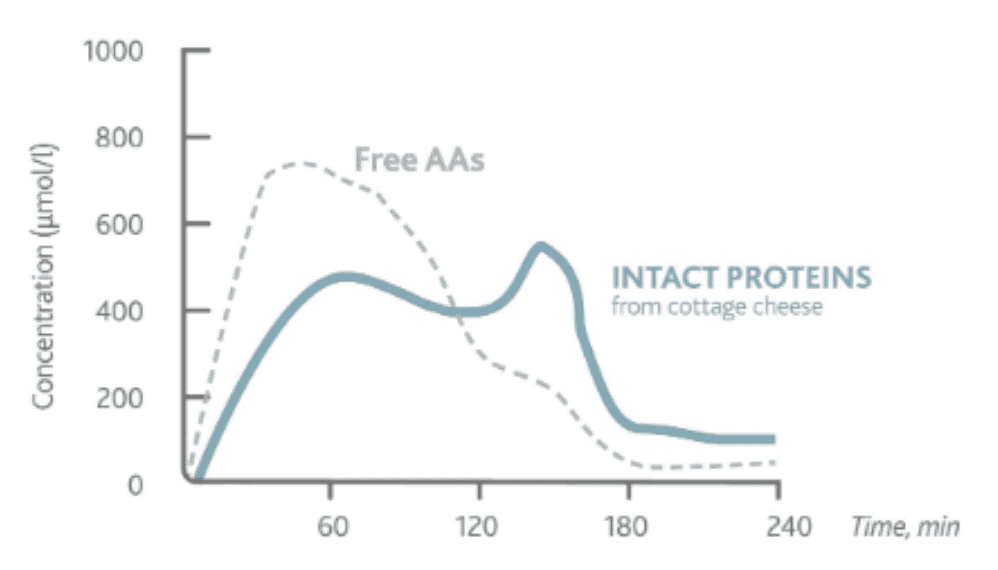
Phenylketonuria (PKU) is an autosomal recessive disorder caused by a deficiency in the enzyme phenylalanine hydroxylase (PAH). PAH is a tissue-specific enzyme that is expressed in humans and converts phenylalanine (Phe) to tyrosine with the aid of tetrahydrobiopterin (BH4). Tyrosine is used to make neurotransmitters, melanin and broken down to produce energy. Gene mutations reduce the activity of PAH. Most of these mutations cause aberrant folding of proteins or disrupt the way the gene's instructions are used to make PAH. Deficiency of PAH results in the autosomal recessive disorder PKU. Failure of Phe catabolism leads to accumulation of Phe and its associated metabolites to toxic levels. High levels of Phe (>1000 μM) affect myelination of neurons that negatively impact cognitive function. Neuronal cells are particularly sensitive to high levels of amino acid resulting in neurotoxicity which results in an increase in neuropsychiatric symptoms.
Exhibit X - Kinetics of Amino Acid in Plasma39
Click image to view larger
Treatment
Similar to many rare diseases, PKU occurs early in infancy and patients benefit from early diagnosis. Since PKU has not been cured, treatment emphasizes a very restrictive diet which includes low-protein, high-starch natural foods which are consumed in small amounts. Following the diet could potentially cause nutritional deficiencies, especially vitamin D and B12. Further, due to the severe limitation of protein intake, PKU patients normally require supplements of L-amino acids, vitamins and nutrients or alternative dietary supplements. Supplements free of Phe have lower biological efficiency. Protein substitutes that mimic physiological absorption kinetics of natural proteins and prolong the absorption of amino acids help in addressing the shortcomings of the condition. Tyrosine supplementation is key to treatment as a majority of Phe is converted into tyrosine under normal conditions. However, compliance remains poor in adolescents and young adults.
There are currently few treatments available in the market for PKU. BH4 is a naturally-occurring compound that serves as a cofactor for PAH. In December 2007, Kuvan (sapropterin dihydrochloride), a synthetic form of tetrahydrobiopterin (BH4), received marketing approval from the FDA making it the first specific therapy for the treatment of PKU patients. BH4 is believed to reduce Phe serum levels by improving the folding conformation of certain types of mutant PAH molecules thereby increasing Phe catabolism. Kuvan is recommended to be used in conjunction with a Phe-restricted diet. Another treatment is Palynziq (pegvaliase-pqpz), an injectable, pegylated, Phe-metabolizing enzyme (phenylalanine ammonia-lyase or PAL) indicated to reduce blood Phe concentrations (<600 µmol/L) in adult patients. The FDA approved Palynziq in 1H 2018. Large neutral amino acid (LNAA) therapy aims to decrease the amount of Phe entering the circulation and the brain. Since high plasma concentrations of LNAAs may block the transport of Phe into the brain, increasing blood LNAA concentrations may reduce uptake of Phe into the brain. It is only recommended for older teens and adults; however, lifelong supplementation is required to maintain normal levels of Phe in blood.
Therapies under investigation
PKU patients could benefit from the use of probiotic to deliver the PAL enzyme to the intestine. Synlogic Inc. is currently conducting clinical trials of SYNB1618, a modified probiotic, in people with PKU to establish safety and tolerability following single and multiple doses. Results are expected in mid-2019. SYNB1618 has received FDA fast track designation.
Gene therapy and enzyme replacement or substitution therapy have yielded more promising data for PKU in recent years. In gene therapy, a functional copy of the PAH gene is delivered to the liver. Enzyme substitution therapy substitutes the activity of an enzyme for the deficient PAH enzyme in PKU. The enzyme substitution allows Phe to be broken down, thereby decreasing blood Phe levels. A promising line of research involves replacing some of the defective liver cells with those that have functional PAH gene.
AGT’s LV - Therapeutic lentivirus expressing phenylalanine hydroxylase for genetic modification of liver
Most of the currently available therapies aim to address the 25% of the PKU population who have a mild case of PKU (hyperphenylalaninaemia). For the majority of patients suffering with severe symptoms (classical PKU), identifying successful treatment has remained futile.
The human PAH gene is uniquely difficult to express at high levels and is a major obstacle to PKU therapy. Researchers at AGT have vigorously pursued the design of the PAH gene to significantly increase its expression in the liver cell. The company is developing AGT323 to replace the defective PAH gene. It is a third generation SIN viral vector that carries a functional copy of the PAH gene and also carries an inhibitory RNA to suppress the expression of mutant PAH gene that is prone to aberrant folding. AGT323 is designed to directly transduce liver cells to restore the normal metabolic pathway in PKU.
Preclinical studies
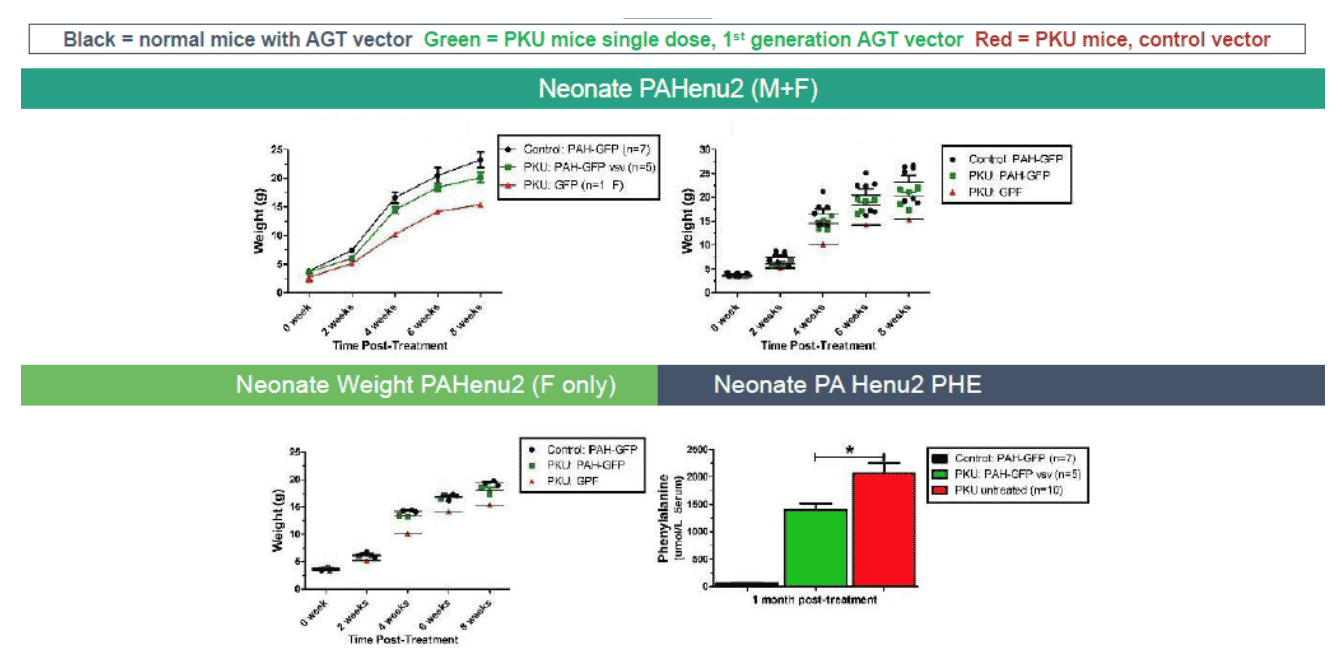
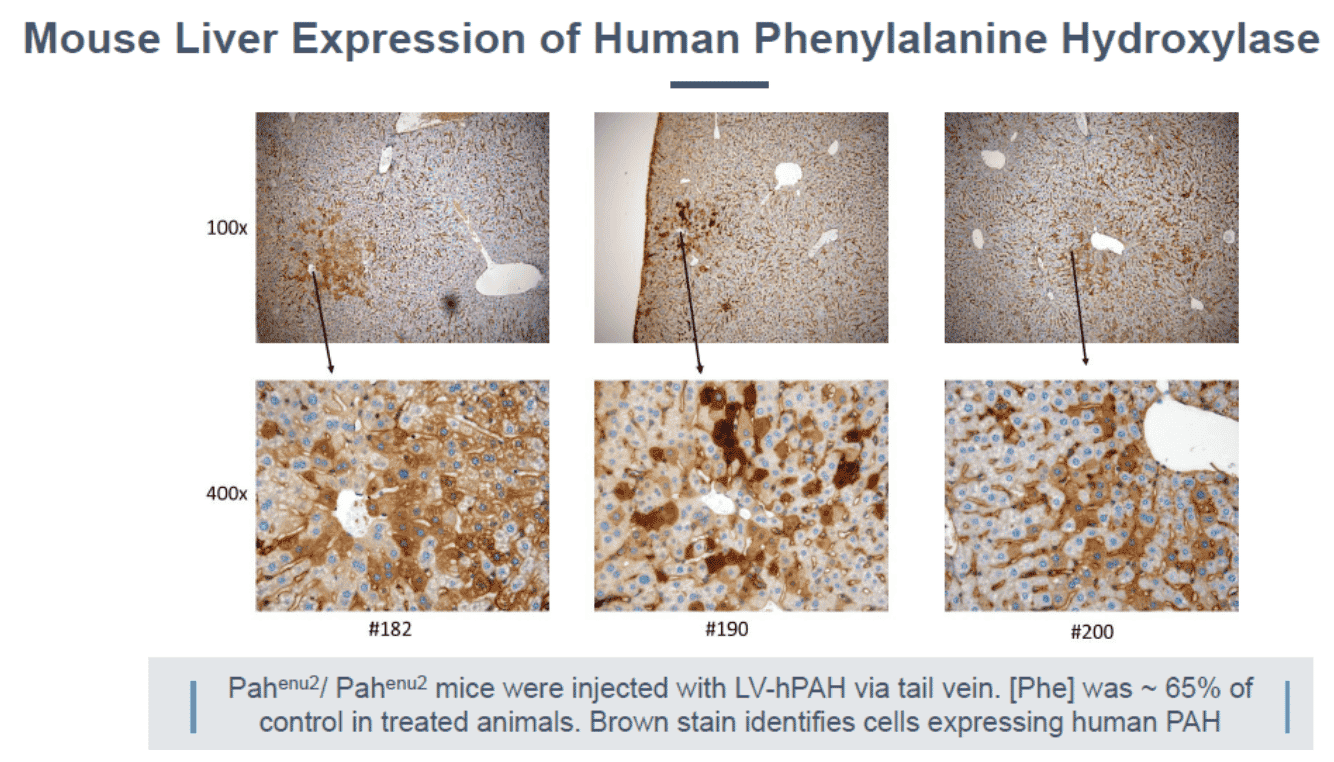
The PKU mouse is an established model of human PKU and exhibits clinical characteristics similar to PKU patients, including hyperphenylalanemia, hypopigmentation, cognitive defects, audiogenic seizures, and maternal PKU syndrome40, 41. At AGT, investigators have evaluated several recombinant LVs carrying the PAH gene to reduce blood Phe levels in the PAHenu2 mouse. Investigators had given several LV vectors into female PAHenu2 mice via the portal vein and monitored blood Phe. Plasma levels of phenylalanine in homozygous (HMZ) PAHenu2 mice were greater than those of heterozygous (HTZ) littermates. In treated PKU mice (5/22), blood Phe levels were below 1500 μM/L. In the untreated group (10/22), average plasma Phe levels were above 2200 μM/L. Histology revealed high levels of expression of PAH enzyme in juvenile mice treated with AGT323.
Exhibit XI - Phe Levels in Mice Using AGT Candidate42
Click image to view larger
Exhibit XII - Mouse Liver Expression of Human PAH43
Click image to view larger
Clinical development plan
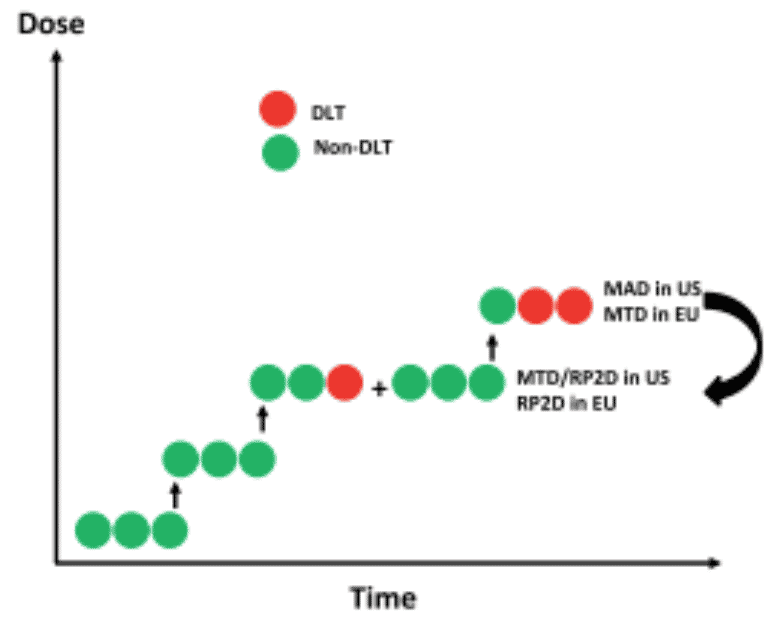
AGT anticipates conducting a Phase I study in humans to determine the safety and efficacy of the LV gene therapy for PKU. Immuno-oncology agents can inadvertently activate the immune system against self, resulting in significant immune-related adverse events and potentially weakening therapeutic benefit. Therefore, it is critical to design clinical trials appropriately. The company plans to have a dialogue with the FDA to design dose escalation studies. Since the portal vein has access to two thirds of the liver, the company plans to administer the viral vector via this route for efficient delivery. The company is prepared to conduct a traditional 3+3 dose escalation study (3 subjects are treated per dose level) to determine maximum tolerated dose. Secondary objectives include recording blood Phe levels and quality of life indicators.
Exhibit XIII - Standard 3 x 3 Dosing Protocol44
Click image to view larger
AGT’s Viral Vector Advantages
This treatment modality deserves attention as it has the potential to permanently cure the disorder while improving cognitive function and quality of life in patients with PKU.
- AGT323 is designed to inject vector directly into the liver. Vectors incorporate the Vesicular Stomatitis Virus (VSV) envelope glycoprotein G that binds to the LDL receptor which is abundant in hepatocytes. This helps in efficient transduction.
- The LV can integrate into target cells thereby rendering long term persistence of the gene expression. This could potentially guarantee a cure with a single therapeutic dose.
- AGT323 has been reengineered to produce in excess of a 10-fold increase in PAH expression. Consequently, the effective dose required is low.
- The LV carries two vectors, one for delivering expression of functional PAH protein in hepatocytes and a small inhibitory RNA (siRNA) for suppressing the expression of mutant PAH.
PKU Upcoming Milestones
Researchers at AGT have established the activity of LV expressing PAH gene as well as suppressing of the mutant gene in preclinical studies. Currently, the team is advancing LV transduction efficiency to achieve proof of concept in a mini-swine model. The company anticipates completion of mouse model studies by the first half of 2019 as it is testing both the highest potency vector and a strategy for pre-conditioning animals to increase hepatocyte transduction rates. They also plan to initiate IND enabling studies in 2019 and possibly submit an application before year end.
PKU Market
PKU is one of the most common monogenic disorders. This debilitating disease is present about 1 in 10,000 – 15,000 births in the U.S. Liver transplantation would be an effective therapy for PKU patients as it would correct the underlying molecular disorder. However, it is not an appropriate alternative for restrictive diet since it is a complex procedure. Vitamin deficiencies are an undesired effect in PKU patients on restricted diet. Clinical manifestation of Vitamin B12 appears several years after inadequate intake of supplements. Vitamin B12 deficiency causes neuropathy, anaemia, dementia and psychiatric states such as depression.
Kuvan has been designated an orphan drug in the U.S. and EU. Kuvan received approval in the EU by end of 2008, Canada in April 2010 and the U.S. in 2013. In 2015, Biomarin acquired the global marketing rights to Kuvan and garnered over $400 million in sales in 2017 from roughly 2,300 patients taking the drug45. The percentage of patients responsive to BH4 therapy using Kuvan range from 20% to 62%. Although BH4 therapy has shown promise, only a small proportion of PKU patients benefit from this treatment. Maternal PKU syndrome still needs attention in managing this condition as there is insufficient safety data from pregnant women who have been taking Kuvan. Newborn screening programs identify children affected by PKU and treatment begins as early as two weeks of age with dietary restriction. Close monitoring keeps symptoms at bay and the child develops with normal intellect. LNAA therapy is only recommended for adult PKU patients who do not adhere to the diet. LNAA supplementation alone or LNAA in combination with a low-Phe diet has shown positive outcomes but long-term effects are not completely known. In 2018, the FDA granted approval to market Palinziq and net product revenues were in excess of $12 million.
AGT’s PKU candidate has potential advantages and could command premium pricing as it promises a cure as opposed to lifelong therapy. AGT’s candidate received an Orphan Drug Designation (#DRU-2018-6572) from the FDA in 2018. This would provide seven years of market exclusivity to AGT’s candidate if commercialized after biologic approval is granted.
Corporate Details
Going Public
AGT is currently structured as a closely held private company but is exploring the process to become public. While timing has not yet been determined regarding any public offering that may occur, we anticipate that details will materialize over the next several quarters. AGT boasts an impressive patent estate, three development programs in HIV, PKU and Immuno-Oncology, two platform technologies in ImmunoTox and Transient Vector technologies and other discovery programs. In February 2019 AGT opened a new round of financing at $5.00 per share with 47.3 million fully diluted shares issued or reserved, suggesting a pre-money valuation of approximately $240 million.
Platform Technology
AGT’s platform is based on the use of viral vector that is able to specifically target a desired critical mechanism of action relevant to a broad cross-section of diseases. The company identifies suitable targets by applying its key criteria which seeks a disease with:
- A precise diagnosis and established natural history
- A quantitative marker available to measure efficacy
- An unmet need and material impact on human population
- Characteristics amenable to gene therapy
AGT’s platform approach seeks indications that leverage AGT’s proprietary experience, knowledge and intellectual property in viral vectors. The company’s objective is to develop tools that will allow the viral vectors to insert corrected genes inside the cells that lack them. The goal is to find drug candidates with a predictable clinical path and that have a defined unmet need in the market in the areas of infectious disease, inherited disease and cancer. They also seek indications with the largest markets when evaluated in context of risk, competition and probability of success. This screening process led to programs in HIV, liver cancer and PKU.
Importance of Time and Cost
Zacks consistently highlights the high cost of therapy as one of the greatest risks to continued growth in the biotechnology and pharmaceutical industries. Drug price inflation has been dramatic over the previous 20 years and new drugs, especially biologics can cost from $150 to $500 thousand per year or per treatment. We see a few components that underlie this reality: high regulatory burden, low success rates and lengthy drug development. AGT’s platform attempts to address all of these challenges with well-understood components to its therapy, extensive bench work that increases the likelihood of success and rapid testing and evaluation processes that can provide a go or no-go indication early in the development process. The drug design process is also more efficient as AGT creates the clinical trial protocol and vector design simultaneously, yielding safer clinical trials and a more potent drug.
The company’s long term strategy is to create a platform that has modular components that can fit together to cure genetic diseases. When viewed from the perspective of rewriting defective software in the human genome, the pathway to a faster and more economical approach to therapy is easier to frame. If successful, AGT may be able to rewrite any genetic code and potentially cure the more than 7,000 monogenic diseases that exist.
Stakeholder's Interests
Gene therapy has attracted interest not only from patients and payors in recent years, but also from regulatory authorities. While innovative drugs and therapies are a boon to mankind, in many cases they also come with exorbitant cost. While the FDA does not have control over prices, they can impact competition to some extent by streamlining the drug approval process. In this effort, former FDA Commissioner Scott Gottlieb and the Director of the Center for Biologics Evaluation and Research Peter Marks have made an effort to increase the number of hires in anticipation of processing more than 200 IND applications by 2020.
Further, the regulatory agency is developing guidelines for cell and gene therapy candidates. Regulatory agencies have offered incentives to developers to expedite therapy candidates to market. Particularly, the regenerative medicine advanced therapy (RMAT) designation came into play in 2016. The RMAT designation allows companies to have frequent dialogue with the FDA, including expedited regulatory review on condition that the candidate will treat or cure a disease categorized as a serious condition. In 2017, Scott Gottlieb announced that certain gene therapies that had the potential to modify cells permanently and bring about a sustained therapeutic effect (genetically modified cells) could be awarded the RMAT designation46. The agency also plans to include clinical guidance documents for the development of gene and cell therapy products for inherited blood disorders and specific neurodegenerative diseases. The documents could also provide guidance on how to use an accelerated approval pathway when the therapy cures a disease.
Insurance companies’ business models are set up for chronic ailments that can be treated over a period of time. A paradigm shift has occurred where gene therapies offering a single treatment command a high price but may also provide a permanent cure. This has challenged the payors as they grapple with antiquated payment procedures that look at short timeframes. Payors are interested in understanding the short-term safety and efficacy data as well as the long-term benefit the gene therapy could offer. It is uncertain as to how long the therapeutic benefit will last in certain patient populations and whether or not a single treatment will be sufficient.
Some insurers offer outcomes-based rebate arrangements and are not paid entirely if the treatment proves ineffective while others allow payments to be made in installments. Industry players (Novartis’ Kymriah and Spark’s Luxterna) and advocacy groups offer different contracting models for their therapy. Value-based payments are an alternate choice but depend on metrics such as lifetime burden of the illness, increases in productivity on returning to work and reductions in use of healthcare resources that are yet to be derived from long-term studies. However, as the sector is still in its infancy, such information is not yet available. Consequently, the potential impact on whether it can reduce healthcare costs is not yet established. Further, lack of new HCPCS codes for novel therapies presents another hurdle that affects reimbursement.
Leadership Team
A strong leadership team comprised of veterans in viral immunology and the medical device industry makes up the backbone of American Gene Technologies. With over three decades of business and entrepreneurial experience, Jeff Galvin (CEO), had held various executive positions at several Silicon Valley startups. Several companies under his management were taken public and/or sold to public companies, including one in the medical-technology arena that was sold to Varian, the leading manufacturer of linear accelerators used in cancer therapy. Jeff’s extensive experience will be instrumental in developing and commercializing AGT’s broad viral vector portfolio. C. David Pauza, Ph.D. is the CSO for AGT and Adjunct Professor of Medicine at the University of Maryland Medical School in Baltimore. Prior to joining AGT, Dr. Pauza was Associate Director for the university’s prestigious Institute of Human Virology and Co-Leader of the Greenebaum Cancer Center Program in Viral Oncology. He is an internationally recognized expert in human virology and viral diseases including HIV, arenaviruses, poxviruses and herpesviruses. Neil Lyons is the Executive Vice President & CFO for AGT. Prior to joining AGT, Neil was the CFO for Cleveland BioLabs and RegeneRx Biotherapeutics. Prior to a career in the biotech industry, Neil held several key positions in the telecom/ IT industries with Alcatel, Bell Atlantic (Verizon) and Honeywell. Additionally, AGT has established a Scientific Advisory Board to support corporate efforts in coming years. The sixteen member Board is expected to provide their support on scientific, clinical and regulatory matters relating to AGT’s lentiviral platform.
Conclusion
Evolution has taken place not only in living organisms but also in science and technology. Over the last several decades gene therapy has refined its approach and improved on many of its shortcomings, emerging as one of the safest and potentially least expensive mechanisms to cure a disease. There is exploding interest in the space from large and mid-sized pharmaceutical companies that have increased their investments in gene therapy and have closed numerous billion dollar deals over the last several years. The gene therapy pipeline is growing in the industry with researchers exploring both ex vivo and in vivo approaches. Approximately 59% of gene therapy candidates employ a viral vector for delivery. Of those, AAV and LV lead the list of those being actively used as they have proven safety, low immunogenicity and long-term transgene expression. A third of the gene therapy pipeline consists of candidates in oncology.
In 2017, the FDA approved the use of two CAR-T therapies for blood cancers. Both gene therapies are ex vivo treatments, as the immune cells receiving gene therapy are removed from the body prior to modification with a viral vector. This approach is aligned with FDA guidance and provides a defined path towards approval. Spark Therapeutics has been rewarded with an FDA approval for Luxturna which uses an AAV vector to deliver a gene (RPE65) directly into the eye of patients suffering from a rare disease called RPE65 mutation-associated retinal dystrophy. While AGT was not the first company to obtain an approved gene therapy product, they have developed a platform that may rapidly and cost-effectively move a product from the lab to commercialization more efficiently than any who have come before.
It is an exciting era for life sciences and especially for gene therapy which has traversed a long road solving many of the problems that have appeared since the first in-human successes were achieved almost 30 years ago. We are still in the beginning stages of the efforts in gene therapy to recode the human genome to repair and replace defective genes. Much like fixing software bugs by rewriting code, modifying the genetic AGTC sequence can eliminate the defects that exist in the human genome. AGT could very well be on the vanguard of a software revolution for the next 100 years.
View The Original Article
This page is a replication of the original content. We do not claim it to be our own.
The original article can be viewed by clicking the button below.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $30,000 annually for these services. Full Disclaimer HERE.
1. From 1971 (Intel 4004) to 2018 (GC2 IPU)
2. Schlimgen, Ryan; et al. Risks Associated with Lentiviral Vector Exposures and Prevention Strategies. Journal of Occupational Environmental Medicine. December 2016, 58(12):1159-1166. Illustration by Nicole Wolf.
3. Worgall, Stefan; Crystal, Ronald. Principles of Tissue Engineering, 4th ed. 2014. P. 657 - 686
4. Gag encodes structural proteins. Gag proteins are necessary for the assembly of virus like particles and helping them mature after they are release from the host cell.
5. Pol refers to DNA polymerase enzyme which generates reverse transcriptase, integrase and protease.
6. Env gene encodes a glycosylated polyprotein that generates viral envelope proteins gp12 and gp41.
7. The long terminal repeat (LTR) is the control center for gene expression.
8. VandenDriessche et al., Blood 100:813–822, 2002
9. American Gene Technologies Corporate Presentation.
10. According to the National Institute of Health
11. US Department of Health and Human Services. https://aidsinfo.nih.gov/understanding-hiv-aids/glossary/171/capsid
12. In some rare circumstances the CXCR4 co-receptor replaces CCR5 co-receptor in the process.
13. whatwhenhow.com. http://what-when-how.com/wp-content/uploads/2012/04/tmp10820_thumb_thumb.jpg
14. Video link for HIV infecting CD4+ T cell: https://www.youtube.com/watch?v=odRyv7V8LAE&t=108s
15. The term "AGT103" refers to the lentiviral vector that contains a miR30-CCR5/miR21-Vif/miR185-Tat microRNA cluster sequence. The term "AGT103-T" refers to a cell that has been transduced with a lentivirus that contains the AGT103 lentiviral vector.
16. Hitachi Chemical Advanced Therapeutics Solutions (HCATS) is the cell manufacturing and development platform used by AGT.
17. AGT February 2019 Science and Partnering Presentation. Used by permission of American Gene Technologies International.
18. AGT February 2019 Science and Partnering Presentation. Used by permission of American Gene Technologies International.
19. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat database: incidence (2009–2015).
20. Liver transplantation for hepatocellular carcinoma: extension of indications based on molecular markers
J Hepatol, 49 (2008), pp. 581-588
21. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis, Lancet Oncol, 10 (2009), pp. 35-43
22. Novel advancements in the management of hepatocellular carcinoma in 2008, J Hepatol, 48 (2008), pp. S20-S37
23. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3036307/
24. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunither 2005;28:496-504.
25. www.ncbi.nlm.nih.gov/pmc/articles/PMC5937202
26. Journals.sagepub.com/doi/full/10.1177/1010428317692229
27. Cancer 2013; 120: 229–237
28. Eur J Cancer. 2016;54:139–48.
29. Hepatoma Res 2018;4:51. Immune system in cancer and therapies targeting the various stages in cancer cycle. CTLA-4: cytotoxic T lymphocyte associated antigen-4; PD-1: programmed cell death 1 protein; PD-L1: PD-1 ligand
30. Transl Lung Cancer Res 2014;3(1):23-33
31. www.frontiersin.org/articles/10.3389/fimmu.2018.00800/full
32. J Exp Med 2003; 197 : 163-8
33. J Immunol 2009; 182 : 8118-24.
34. J Immunol 2001; 167 : 5092-8.
35. Expert Opin Biol Ther. 2008 Jul; 8(7):875-83.
36. Cancer Res. 2011 Jul 1; 71(13):4562-72.
37. Blood. 1998 Oct 1; 92(7):2556-70.
38. Located at: https://clinicaltrials.gov/
39. Adherence Issues in Inherited Metabolic Disorders Treated by Low Natural Protein Diets. 2012. Ann Nutr Metab 61:289
40. Mouse models of human phenylketonuria. Genetics 1993;134:1205–1210.
41. Epilepsy in phenylketonuria: a complex dependence on serum phenylalanine levels. Epilepsia 2007;48:1143–1150.
42. Copyright 2019 American Gene Technologies. Used by permission.
43. Copyright 2019 American Gene Technologies. Used by permission.
44. Cancer Control July 2014, Vol. 21, No. 3
45. Source: Evaluate Pharma
46. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm573443.htm