NEWSLETTER VOLUME 12

HIV Program Update
Five for Five!
American Gene Technologies (AGT) announced that it has reached another important milestone in its HIV cure program. Five participants were treated with AGT103-T and are stably engrafted with genetically modified cells.
Laboratory studies confirmed substantial increases in virus-specific T cells consistent with improved immunity against HIV in all participants. The early data addresses key trial endpoints and is aligned with the objective of restoring natural immunity against HIV.
“These latest laboratory studies of clinical material are extremely encouraging,” said AGT CEO Jeff Galvin. “To see these markers in all five trial participants indicates that prospects are bright for a potential one-and-done therapy to functionally cure HIV. This milestone brings us another step closer to our goal of returning people living with HIV to a normal life without the side effects of ART and having no further consequences of HIV.” Read more...
Check out ABC 7's story on our latest results below.
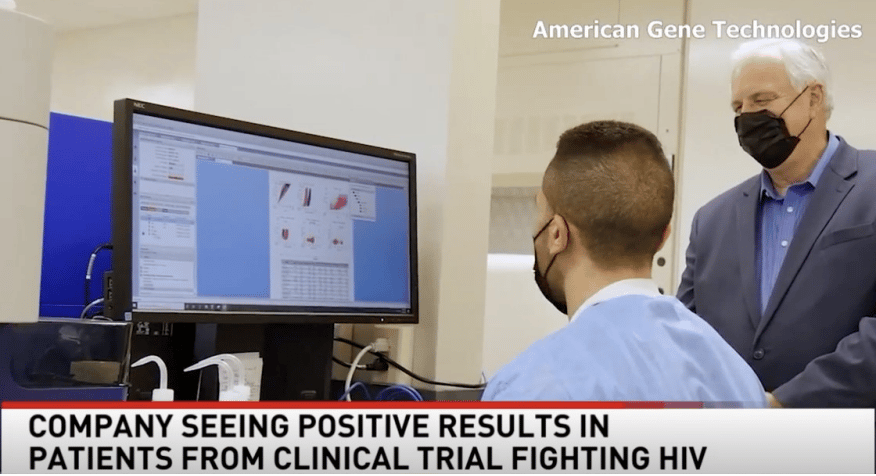
First Participant Infusion
American Gene Technologies (AGT) treated the first participant in its Phase I clinical trial to evaluate safety of the cell and gene therapy product AGT103-T in May 2021.
AGT in the News
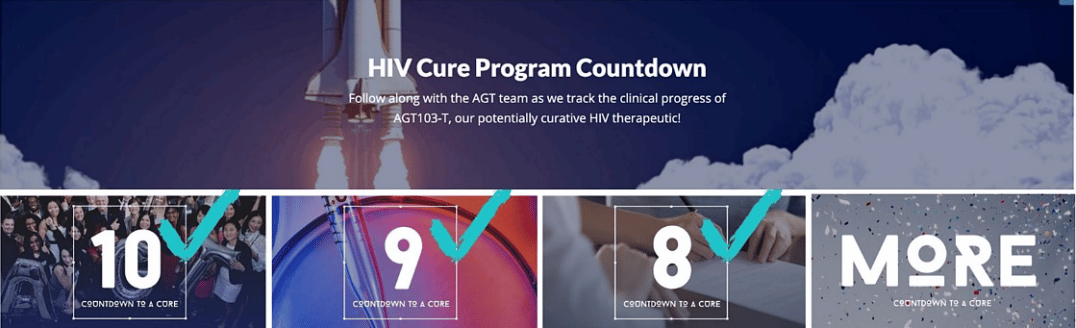
HIV Cure Countdown
Follow along with the AGT team as we track the clinical progress of AGT103-T, our potentially curative HIV therapeutic! Subscribe to this page to get email notifications each time we reach a new milestone:
Check Out AGT's Growing Team of Advisors
AGT Appoints Dr. Drew Palin as Business and Strategy Advisor to CEO
American Gene Technologies (AGT) announced the appointment of Dr. Drew
Palin as business and strategy advisor to Jeff Galvin, CEO. Read more...
American Gene Technologies Appoints Dr. Marcus A. Conant as Special Advisor to the CEO
American Gene Technologies (AGT) announced the appointment of Dr. Marcus A. Conant, a leading dermatologist in the U.S. and one of the first physicians to diagnose and treat AIDS, as special advisor to AGT CEO Jeff Galvin. Read more...
American Gene Technologies Appoints Dr. Robert R. Redfield as Special Advisor to the CEO
American Gene Technologies (AGT) announced the appointment of Dr. Marcus A. Conant, a leading dermatologist in the U.S. and one of the first physicians to diagnose and treat AIDS, as special advisor to AGT CEO Jeff Galvin. Read more...
DNA Valley
Our DNA Valley series highlights cutting-edge cell and gene therapy products, technologies, and platforms in use or in development by leading innovative companies in Maryland. CEO Jeff Galvin interviews industry executives charging forward with strong leadership and knowledge to bring therapies through the clinic and eventually into the market, as well as thought leaders creating opportunities within the booming Biotech industry in Maryland.
Fireside Chat with Rich Bendis, CEO of
BioHealth Innovation, Inc. Watch Now.
John Trainer, CFO of NexImmune, on T cell
technology and Biotech in Maryland. Watch
Now.

Workforce Development in the Booming Biotech Industry with Chris Frew, CEO of WorkforceGenetics. Watch Now.
DNA Valley Circles the Globe! Watch SET News
Taiwan's coverage of our HIV Cure Program
now.
Community Spotlight
Life Sciences Education & Innovation Partnership
AGT is creating opportunities for STEM graduates in Maryland. AGT's has hired several talented UMBC Translational Life Sciences Technology degree graduates from The Universities at Shady Grove. AGT and CEO Jeff Galvin are proud to be part of the Life Sciences Education & Innovation Partnership with Montgomery County Government, University System of Maryland, Montgomery College, and The Universities at Shady Grove.
AGT Appearances At Industry Events
CEO Jeff Galvin presenting at the BIO
International Convention. Watch Now.
CEO Jeff Galvin presenting at the Cell &
Gene Meeting on the Mediterranean.
Watch Now.
Raising Awareness About Rare Disease
Amber Freed and Brittany Stineman are rare disease moms who have gone through incredible lengths to pursue cures for their sons battling rare diseases. Amber has single-handedly driven multiple translational treatments forward in an effort to cure SLC6A1, and Brittany has raised over $2 million to fund the development of a genetic therapy that could save the lives of SMARD patients. Holly Snyder, a genetic counselor from Illumina, joins the first of the series to share valuable knowledge regarding genetic testing for those seeking a diagnosis.
Follow Us on Social Media
Stay updated on AGT's latest news and support our mission by following us on
social media.