Unboxing Biotech Analyst Report: The Foundation Behind a Potential HIV Cure

The exact origins of Human Immunodeficiency Virus (HIV) are not precisely known, but researchers theorize that the virus may have originated in chimpanzees,1and then crossed to humans via blood borne pathway. It is not radically different from many of the other retroviruses that exist in nature such as the avian sarcoma virus or simian retrovirus, but HIV infects humans. While primates have built up immunity to their version of the virus over thousands of years, humans have been exposed for a much shorter duration and few harbor immunity. The earliest verified infection in humans was found in a man who died in Kinshasa, Democratic Republic of the Congo over 60 years ago.2 His blood sample collected in 1959 tested positive for HIV in the late 1990s.
Through commercial routes and the sex trade, HIV spread from the Congo throughout the world where it was largely overlooked until the early 1980s when it emerged in major U.S. cities as a mysterious illness. It was associated with unusual infections such as pneumonia and a certain type of cancer called Kaposi’s sarcoma, which produced highly visible purplish spots on the skin. Acquired Immune Deficiency Syndrome (AIDS), the disease that results from HIV, was first recognized on June 5, 1981. The Center for Disease Control and Prevention’s (CDC’s) Morbidity and Mortality Weekly Report (MMWR) made note of it in conjunction with five cases of pneumocystis pneumonia in Los Angeles in otherwise healthy young men.
In the early days, when little was known and treatment and effective prevention were years away, cases grew quickly. According to the CDC, there was a dramatic rise in U.S. HIV incidence from 1981 to 1986, which then declined over the next several years as better prevention and new drugs such as azidothymidine (AZT) came into use.
Exhibit I – Various HIV Incidence Statistics Among Persons Aged ≥13 Years — United States, 1981–20083
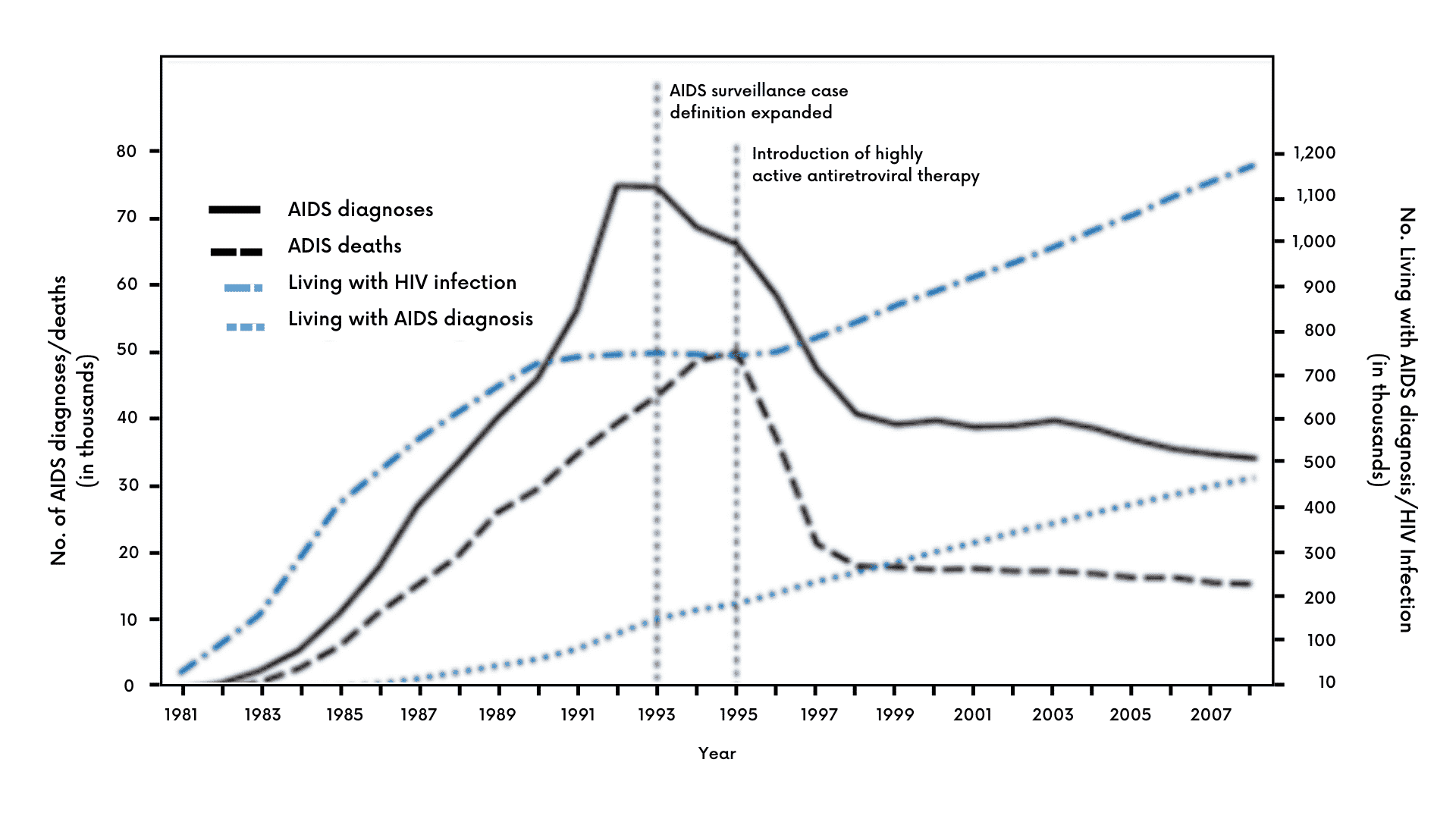
How Common is HIV?
Based on the latest data from the CDC, there are approximately 1.2 million persons in the U.S. with HIV and 13% are unaware of their condition.4 In 2019, an estimated 34,800 new HIV infections occurred in the U.S., a decline from the 37,800 estimated in 2015.5 In the global sphere, the World Health Organization (WHO) reported that there were 37.7 million people living with HIV around the world in 2020 with an estimated 1.5 million new cases occurring during the year.6
How Does it Infect?
HIV is usually transmitted through sexual activity, blood transfusions, hypodermic needles and from mother to child during pregnancy. After HIV enters the body, it seeks out CD4+ T cells,7 then binds to and enters these cells using the CD4 receptor and the CCR5 co-receptor. Without the CCR5 co-receptor, most HIV would be unable to infect the host. In some cases, HIV may use the alternate CXC chemokine receptor 4 (CXCR4) co-receptor to gain entry to the cell.8 After binding and entry, viral enzymes are then released from the capsid into the cytoplasm, which help the viral DNA integrate into the host cell’s genome. The process continues with the production of viral proteins, which assemble into complete virions that exit the cell to repeat the cycle and go on to infect other T cells.
Since HIV infects and disables the very cells that should be combatting the viral invaders, the body’s immune system begins to fail and allows opportunistic infections to gain a foothold.
Retaliation
The first counterattack against HIV came in the mid-1980s. A drug that had been initially developed twenty years earlier for cancer called azidothymidine (AZT) was designed to integrate counterfeit nucleotides into cancer cell DNA to stop cell growth. As it turned out, the drug interfered with the enzyme reverse transcriptase, which could prevent a virus from replicating.
Exhibit II – Combination Therapy Truvada9
Many antiviral drugs gained marketing approval by the early 2000s and were combined in a single tablet that allowed patients to reduce the considerable number of pills previously required. Other therapeutic interventions such as PrEP, or pre-exposure prophylaxis, substantially reduced an individual’s risk of getting HIV if exposed. While many new antiretrovirals (ARVs) and combinations thereof helped control a patient’s HIV and prevented the infection of others, they did not provide a cure. Another shortcoming of ARVs is the side effects. While these have improved dramatically over time, still many remain which can have a long-term negative impact on a patient’s life. Perhaps the most salient impacts of ARVs are the daily burden and cost; however, there are other physical symptoms that also weigh heavily on patients.
Finding an Imperfect Cure
To address the shortcomings of existing therapy, pioneers in the space speculated that they could block HIV’s spread by transplanting an HIV-protected immune system into a patient. The first success in permanently suppressing HIV was achieved in 2008 with a bone marrow transplant from a donor harboring a favorable genetic mutation.10 The high-risk transplant was primarily performed to treat the patient’s blood cancer; however, it also strengthened the immune system to avoid HIV infection by introducing T cells that lacked the CCR5 co-receptor. Several procedures using this approach have been successful, but the expense, risk and primary use for cancer treatment limits it to a small subset of patients. Safer and more repeatable efforts have been made using a gene editing process that mimics the stem cell transplant technique. We look at two trailblazing pursuits that provided a foundation for future programs.
Sangamo
Sangamo, a California-based research company, used gene editing in its effort to cure HIV. The approach employed enzymes called zinc-finger nucleases to modify DNA to potentially cure the viral infection by removing the CCR5 co-receptor from immune cells. The process withdrew T cells from an antiretroviral therapy (ART)-treated HIV patient, modified the cells in the lab to resist HIV infection, then returned the T cells to the patient so they could go about their normal business and eradicate the virus. Four weeks after infusion, ART was withdrawn. As hoped, T cells initially increased and modified T cells declined at a slower pace than native T cells. The trial was a partial success, with two patients able to control HIV without ART for over a year and one of them able to attain durable suppression.
Calimmune
Another gene therapy method advanced by Calimmune11 was similar to the Sangamo approach. It sought to reduce CCR5 expression through the use of RNA interference and a fusion inhibitor. Conditioning was used prior to treatment to clear the viral reservoir; however, none of the patients that completed treatment were ultimately able to control the virus without the help of ART.
Despite the lack of a cure, the research provided a useful foundation for others. The primary learnings include the importance of targeting HIV-specific T cells for CCR5 receptor deletion, attacking HIV’s replication processes from multiple directions and transducing and reinfusing an exponentially larger number of HIV-protected T cells in the patient than was previously done.
Why Is HIV So Insidious?
HIV has developed the ability to attack the very type of cell that should detect and kill it. This is the HIV-specific CD4+ T cell, which is normally the general in charge of the body’s immune army. When HIV enters the mix, it binds to the CD4+ T cell and injects its viral RNA, which subsequently integrates into the T cell’s DNA, preparing for further replication. The virus may remain dormant for years until the infiltrated cell becomes activated. After activation, the viral components of a new HIV virion are formed. They then assemble and bud off from the T cell to repeat the cycle until the vast majority of all T cells are infected.
Exhibit III – HIV Infection in Target T Cells12
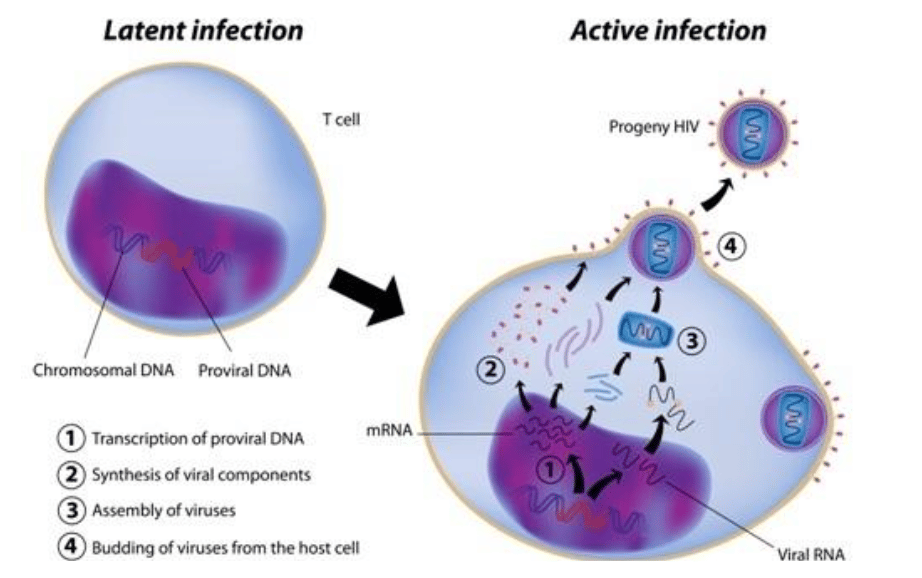
HIV’s cunning nature derives from its ability to infect helper (CD4+) T cells. T cells are critical elements of the immune system that are responsible for coordinating killer (CD8) T cells and antibody-producing B cells. HIV is able to spread from helper T cell to helper T cell, eventually killing enough of them to impair the immune system’s ability to coordinate an immune response, leaving the host open to a wide variety of bacterial and viral infections. After a period of time without treatment, the individual becomes immune deficient and the patient will die of opportunistic infections or virus-causing cancers.
AGT103-T
Building on the shoulders of predecessors’ work with hematopoietic stem cell transplants and gene therapy, American Gene Technologies® has designed a family of lentiviruses to tackle HIV. American Gene™ core technology in viral vectors was cultivated by National Institutes of Health (NIH) biochemist Dr. Roscoe Brady. In 2007, technology entrepreneur Jeff Galvin collaborated with Dr. Brady to undertake the preclinical work necessary to develop the library of viral vectors that would be able to address the indications in the company’s pipeline. Using a software analogy, the process is akin to reprogramming corrupt human genes and overwriting defective genetics thereby debugging a faulty genome.
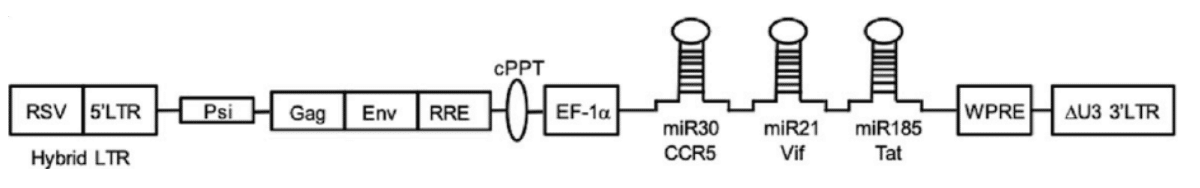
Lentivirus Development
AGT10313 is a third generation, self-inactivating lentivirus vector carrying three inhibitory RNAs. The recombinant lentivirus vector encodes microRNAs:
- miR30 CCR5,
- miR21 Vif, and
- miR185 Tat.
The miRNA neutralizes the normal function of the various elements of HIV. The first, miR30 CCR5, deletes the CCR5 receptors that appear on the CD4+ T cell’s surface and allows HIV entry. Without the receptor, HIV will not be able to infect and disable the cells that fight it. The second, miR21 Vif, or virion infectivity factor, reduces the ability of HIV to infect by blocking the Vif protein. The third miRNA, miR185 Tat, disables Tat or Trans-Activator of Transcription, which reduces the efficiency of viral transcription. The Vif and Tat miRNA also protect the CD4+ cell from the less common form of HIV that uses CD4 and CXCR4 proteins for attachment and entry. The three-pronged attack by the microRNA silences the activity of the associated genes, enabling the CD4+ T cells to methodically eliminate HIV at full strength.
Exhibit IV – Lentivirus Vector AGT103-T14
Once the lentivirus vector is ready, the process of delivering it to the appropriate cells begins. White blood cells are removed from a patient with HIV through leukapheresis. The cells are then modified in the lab using a five-step process including peripheral blood mononuclear cell (PBMC) isolation, peptide activation of Gag-specific cells, non-target cell depletion of CD8, CD5 and γδ T cells, lentivirus vector transduction and Gag-specific CD4 T cell expansion.
Exhibit V – AGT103-T Delivery Process15
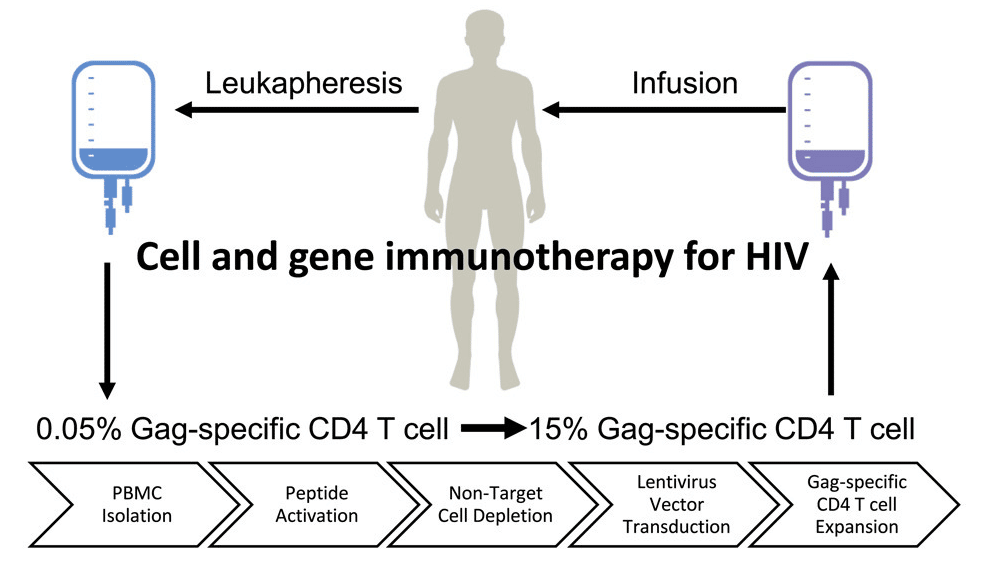
Following the preparation of AGT103-T and sufficient expansion of the HIV-specific CD4+ T cells, AGT103-T is infused into the patient. The modified T cells are now immune from HIV infection and can find and clear the virus from the patient. The healthy T cells will now be able to direct the immune system and the actions of natural killer (NK) cells, CD8+ T cell response, memory cells and the critical role of immune suppression.
AGT103-T reconstitutes autologous HIV Gag-specific16 CD4 T cells by modifying them to be immune to future HIV infection, then expanding them in culture. The modified cells, increased nearly 1000-fold, are then reintroduced to the HIV patient. The cells are resistant to infection by HIV and are able to coordinate an immune response against HIV, directing the immune system to advance throughout the body, recognizing and killing cells that produce HIV. The immune system’s war of attrition on the viral reservoir drives decay of HIV in the body to a level where it is undetectable,17 even when measured by a sensitive laboratory test. One of the hurdles that has stalled previous attempts to find a gene therapy HIV cure has been growing and infusing a sufficient number of HIV-specific CD4 T cells. AGT103-T seeks to address this weakness by dramatically expanding the number of Gag-specific cells.
The Trial
While AGT103-T’s roots extend back more than a decade, preparations for evaluating AGT103-T in the clinic escalated in early 2018 with the completion of the cell processing protocol, an integral component of the investigational new drug (IND) application that would be submitted to the FDA. Other parts of the application, including cGMP18 vector manufacturing, animal toxicology, process qualification activities, and cell process, development and testing, were completed in 2019. The IND was submitted to the FDA in October 2019, and, after an extended period of addressing agency questions, clearance to begin human trials was granted in August 2020. This kicked off the trial RePAIR, shorthand for Restore Potent Antiviral Immune Responses.
RePAIR is a Phase I study to assess the safety of AGT103-T for autologous donor lymphocyte infusion in HIV+ patients with viremia under control using ART. It initially planned to enroll six participants with the option to increase up to 18 and is now evaluating seven subjects. Endpoints for the Phase I trial are primarily oriented towards safety, but secondary outcomes include blood markers of efficacy and maintenance of viral control after removing ART. Trial design allows for male and female patients from 18 to 60 years of age that are able to suppress HIV to less than 50 copies of viral RNA per mL of plasma for two years using ART. The patients must be otherwise healthy.
HIV Under Control: So What’s the Problem?
The combination antiretroviral therapy that emerged in the late 1990s was a giant leap forward for those with HIV at the time. It radically changed the prognosis for the disease from one of almost certain death, to one where a near-normal lifespan could be expected and where HIV can almost be considered a chronic disease. Despite the dramatic improvement in expected outcomes, ART side effects can be unpleasant and the bar for acceptable treatment 35 years later is now higher. Short-term effects include nausea and diarrhea. Long-term impacts involve bone demineralization, heart disease, liver and kidney problems, increased cancer risk and early aging.
While ART has been life changing and life extending for HIV patients, there is a darker side to the medication that emerges in the side effect profile of the drug class.19,20 Mitochondrial toxicity,
hypersensitivity, liver and cardiovascular-related events are among the side effects suffered by patients on ART.
Mitochondrial toxicity, which manifests itself in myopathy, neuropathy, hepatic steatosis and lactic acidemia, are consequences of using ARTs and can begin to appear within days of beginning the therapy. The most serious mitochondrial toxicities include lactic acidosis and pancreatitis. Hypersensitivity to ART can lead to rash, pruritus, fevers and mucosal ulceration among other less common features.
Even though ART provides a substantial improvement in quality of life and survival, the side effects represent a tremendous burden. When combined with the stigma of having a deadly virus in your system, the weight of a never-ending pill regimen and its ongoing cost, a cure becomes the logical next step for meaningful improvement in quality of life for an HIV patient.
RePAIR Treatment Interruption
AGT103-T is the subject of a Phase I trial; a stage which is chiefly focused on a medicine’s safety profile. RePAIR added another component to its study goals to measure the effects of treatment interruption. This modification will allow investigators to determine whether or not the gene therapy will provide durable suppression and potentially eradicate HIV from the patient. Treatment interruption is not recommended outside of strictly controlled clinical trials or direct oversight by a physician, as the virus may proliferate and the patient may be at risk of opportunistic diseases, cardiovascular problems and death.21 The patient may also see a surge in viremia and could spread the virus with close contacts.
Despite the risks, preclinical work and blood markers of efficacy provide strong support for the success of American Gene’s HIV therapy. In a February 2022 interim update, investigators for the RePAIR trial reported that five patients were stably engrafted with genetically modified cells. Furthermore, HIV-specific T cells were higher in patients’ blood compared with pre-infusion metrics.
In late June 2022, the principal investigators for the study announced that treatment interruption for the seven subjects enrolled in the trial was approved. Trial managers anticipated that the first interruptions of ART would begin the next month. For the average person living with HIV, it generally takes from four to eight weeks after a patient stops ART for the viral load to rebound22,23,24,25, so it is expected that in autumn, the first signs of durable control may emerge. If some or all of the subjects are able to control their viremia several months after halting ART, it will be a strong sign that AGT103-T has achieved a notable success, elevating it to the status of a triumph comparable with the cure for hepatitis C a decade ago.
What Does the Future Hold?
Within several weeks of the start of treatment interruption, investigators should have a sense of the ability of AGT103-T to control HIV without ART. This is an exciting time for HIV research building on other breakthroughs that have been made. AGT103-T benefits from prior learning and in short order will demonstrate whether or not it can address this 40-year quest to liberate HIV patients from this dreadful affliction.
1Sharp, PM; Hahn, BH. The evolution of HIV-1 and the origin of AIDS. Philos Trans R Soc Lond B Biol Sci. 2010 Aug 27;365(1552):2487-94. doi: 10.1098/rstb.2010.0031.
2Zhu TF, et al. An African HIV-1 sequence from 1959 and implications for the origin of the epidemic. Nature. 1998; 391:594–597.
3CDC Data. Morbidity and Mortality Weekly Report, HIV Surveillance, United States, 1981 – 2008.
4CDC data, hiv.gov, U.S. Statistics accessed July 2022.
5HIV.gov U.S. Statistics. Accessed June 2022.
6HIV/AIDS Global Statistics. World Health Organization, The Global Health Observatory. Accessed June 2022.
7These are T cells expressing the cluster of differentiation (CD) 4 receptors.
8Binding to CCR5 is known as CCR5 (or R5) tropism, binding to CXCR4 is known as CXCR4 (or X4) tropism, and the ability to bind to either coreceptor is described as dual tropism
9Source: Shutterstock.
10See our previous article entitled ARC of HIV History 2 – A Beginning and an End, under the heading At Last, What We’ve Been Waiting For, to read a summary of the first stem cell transplant that cured an HIV patient.
11Calimmune was later acquired by CSL Behring,
12Source: Shutterstock.
13AGT103 is the lentiviral vector that is transduced into HIV-specific T cells. After the transduction, the gene therapy is then designated AGT103-T.
14Li, H. et al. Preclinical Development and Clinical-Scale Manufacturing of HIV Gag-Specific, LentivirusModified CD4 T Cells for HIV Functional Cure. Molecular Therapy, Methods & Clinical Development published May 3, 2020.
15Li, H. et al. Preclinical Development and Clinical-Scale Manufacturing of HIV Gag-Specific, LentivirusModified CD4 T Cells for HIV Functional Cure. Molecular Therapy, Methods & Clinical Development published May 3, 2020.
16The Gag protein appears on HIV and is the antigen targeted by the CD4+ T cell.
17The undetectable threshold is biologically significant, as individuals meeting this boundary do not transmit HIV.
18Current Good Manufacturing Practices (cGMP)
19 Carr, A., Cooper, D.A. Adverse Effects of Antiretroviral Therapy. The Lancet, October 21, 2000.
20Reisler RB, Han C, Burman WJ, Tedaldi EM, Neaton JD. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr 2003;34:379-86.
21El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296.
22nejm.org/doi/full/10.1056/NEJMoa1608243
23https://pubmed.ncbi.nlm.nih.gov/26588174/
24https://journals.lww.com/aidsonline/fulltext/2019/04010/clinical_trials_of_antiretroviral_treatment.3.aspx
25Interview with HIV clinician and expert Dr. Marcus Conant.
26Sofosbuvir was approved as a first-in-class nucleotide drug to treat hepatitis C in December 2013, and was a radical improvement over treatments with low cure rates and very unpleasant side effects.





