After the first three participants in the HIV cure human trial showed no serious adverse events, the DSMB allowed acceleration of the trial.
Our Vision for an HIV Cure
HIV Cure Project Initiated: October 2016. AGT meets with the FDA and defines a path forward for AGT103-T. FDA outlines safety requirements and greenlights further development.
IND Submission: October 2019. The Investigational New Drug (IND) application provides preclinical data and a rationale for why this unique immuno-regulatory approach is worthy of advancing into the clinic.
IND Approved - Clinical Trials Begin: August 2020, FDA approves the IND and clears AGT to begin a Phase 1 human trial.
First Patient Enrolled - October 2020. AGT begins the Phase 1 trial for AGT103-T, and the first trial participant is expected to be infused early in 2021.
Initial Safety Data - July 2021. The first participant experiences no adverse events; USMB reviews safety data and approves continuation of study.

Watch clips from AGT's red carpet event celebrating its IND submission to the FDA.
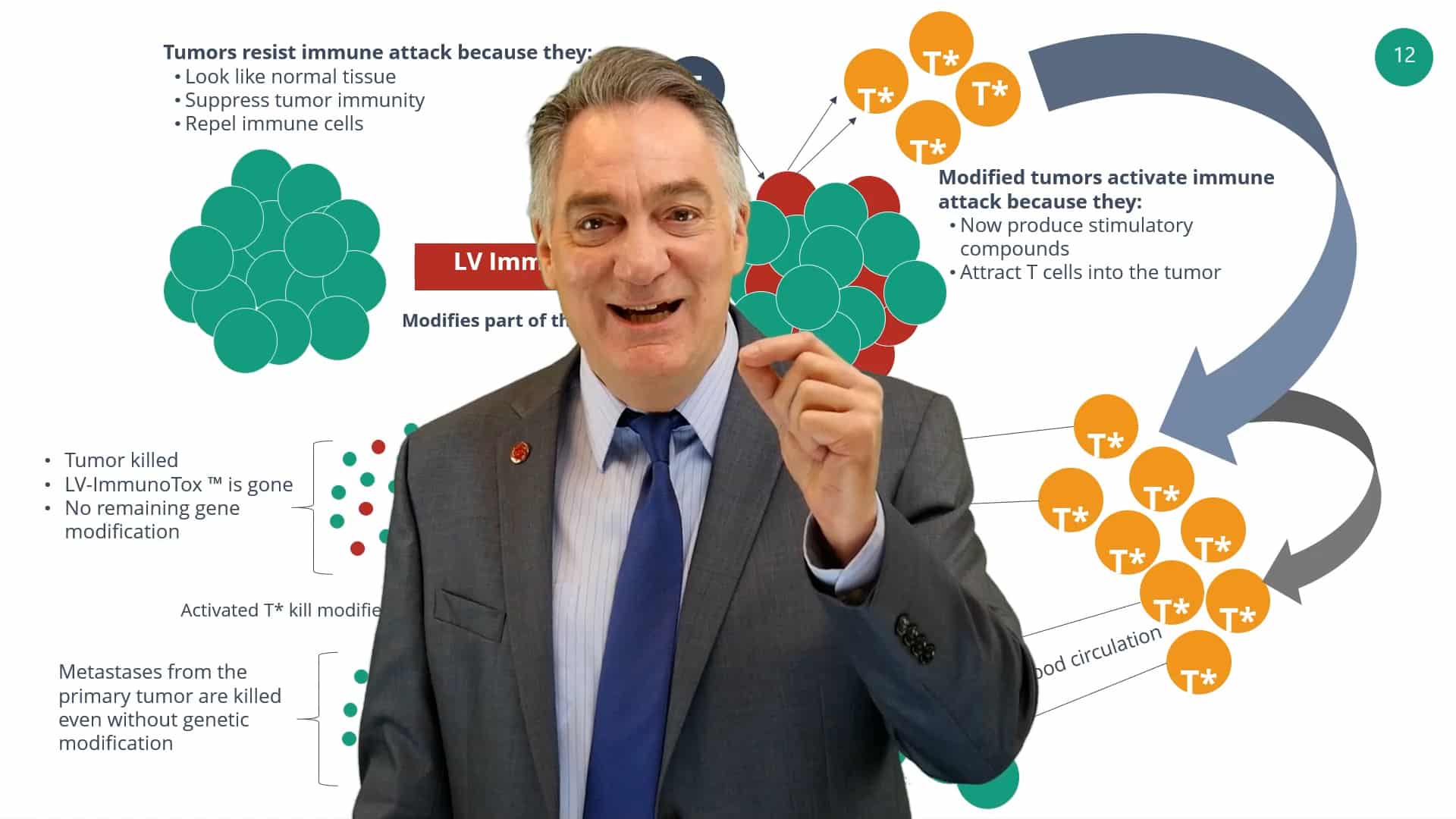
Cutting Edge Methods to Kill Cancer
AGT’s innovative immuno-oncology program aims to create genetic drugs that increase treatment potency, broaden the range of cancers treated, and provide the greatest value for patients. This led to the development of the ImmunoTox program, a genetic medicine introduced by a lentiviral vector delivered to sites of solid tumor growth where it penetrates tumor cells and alters their normal metabolism. The ImmunoTox program’s novel approach causes the build up of a small molecule danger signal that activates γδ T cells to kill cancer cells.
Who We Are
American Gene Technologies International Inc. (AGT) is a private biotechnology company pursuing cures and treatments for infectious diseases, cancers and monogenic disorders.
AGT's programs are designed to:

What We Do
For over a decade, AGT has developed, tested and banked thousands of lentiviral vectors that can be adapted to the specific needs of new target diseases. AGT has developed gene therapy for diseases using this rapid gene delivery platform.
Gene Therapies
AGT’s platform allows it to pursue clinical “cures” for complex diseases. The company has developed patent-protected gene therapeutics that are breakthroughs in medicine.
GENE THERAPY
HIV/AIDS
Immunotherapy for HIV disease with gene modified T cells
IMMUNO-ONCOLOGY
Activates innate T cell immunity capable of killing all tumor cells with its vector used in immuno-oncology
GENE THERAPY
Phenylketonuria (PKU)
Treats PKU by bringing a functional gene into cells that express a dysfunctional gene due to mutation
Leadership Team
American Gene Technologies™ (AGT) is building a rich product pipeline based on its lentiviral vector platform. Our three leading therapeutics are well into the laboratory phase and scheduled to move into the clinical phase over the course of the next 6-18 months. AGT is in the process of testing and commercializing genetic medicines that will cure or halt the progression of several major diseases.
-

Jeff earned his BA degree in Economics from Harvard in 1981. He has more than 30 years of business and entrepreneurial experience including founder or executive positions at a variety of Silicon Valley startups.
Jeff Galvin
CEO and
Founder
-
David has 35 years of experience in HIV, immunology, virology, and cancer biology, and has held faculty positions and at University of Wisconsin and University of Maryland medical schools.
C. David Pauza, PhD
Chief Science
Officer
-

Andrew has more than 20 years of experience in accounting, finance, strategy, raising capital, and corporate governance in both private companies and non-profit organizations.
Andrew Miller, CPA
Vice President
of Finance
-
Former Secretary of the United States Department of Health and Human Services, worked fervently to modernize healthcare delivery as the former Governor of Wisconsin.
Tommy Thompson
Director
and Senior Advisor
-

Drew has many years of experience as a physician start-up executive bringing new technologies to market to improve patient experience and outcomes.
Drew Palin
Business and Strategy Advisor
to the CEO
-

Dr. Conant was one of the first physicians to diagnose HIV in San Francisco while running the inpatient dermatology service at the University of California San Francisco.
Marcus Conant
Special Advisor
to the CEO

AGT's Core Objectives
AGT Press Releases
Sorry, we couldn't find any posts. Please try a different search.
Sorry, we couldn't find any posts. Please try a different search.